CHAPTER 8
ELECTRODES AND FILLER METALS
Section I. TYPES OF ELECTRODES
8-1. COVERED ELECTRODES
a. General. When molten metal is exposed to air, it absorbs oxygen and nitrogen, and becomes brittle or is otherwise adversely affected. A slag cover is needed to protect molten or solidifying weld metal from the atmosphere. This cover can be obtained from the electrode coating. The composition of the electrode coating determines its usability, as well as the composition of the deposited weld metal and the electrode specification. The formulation of electrode coatings is based on well-established principles of metallurgy, chemistry, and physics. The coating protects the metal from damage, stabilizes the arc, and improves the weld in other ways, which include:
(1) Smooth weld metal surface with even edges.
(2) Minimum spatter adjacent to the weld.
(3) A stable welding arc.
(4) Penetration control.
(5) A strong, tough coating.
(6) Easier slag removal.
(7) Improved deposition rate.
The metal-arc electrodes may be grouped and classified as bare or thinly coated electrodes, and shielded arc or heavy coated electrodes. The covered electrode is the most popular type of filler metal used in arc welding. The composition of the electrode covering determines the usability of the electrode, the composition of the deposited weld metal, and the specification of the electrode. The type of electrode used depends on the specific properties required in the weld deposited. These include corrosion resistance, ductility, high tensile strength, the type of base metal to be welded, the position of the weld (flat, horizontal, vertical, or overhead); and the type of current and polarity required.
b. Types of Electrodes. The coatings of electrodes for welding mild and low alloy steels may have from 6 to 12 ingredients, which include cellulose to provide a gaseous shield with a reducing agent in which the gas shield surrounding the arc is produced by the disintegration of cellulose; metal carbonates to adjust the basicity of the slag and to provide a reducing atmosphere; titanium dioxide to help form a highly fluid, but quick-freezing slag and to provide ionization for the arc; ferromanganese and ferrosilicon to help deoxidize the molten weld metal and to supplement the manganese content and silicon content of the deposited weld metal; clays and gums to provide elasticity for extruding the plastic coating material and to help provide strength to the coating; calcium fluoride to provide shielding gas to protect the arc, adjust the basicity of the slag, and provide fluidity and solubility of the metal oxides; mineral silicates to provide slag and give strength to the electrode covering; alloying metals including nickel, molybdenum, and chromium to provide alloy content to the deposited weld metal; iron or manganese oxide to adjust the fluidity and properties of the slag and to help stabilize the arc; and iron powder to increase the productivity by providing extra metal to be deposited in the weld.
The principal types of electrode coatings for mild steel and are described below.
(1) Cellulose-sodium (EXX10). Electrodes of this type cellulosic material in the form of wood flour or reprocessed low alloy electrodes have up to 30 percent paper. The gas shield contains carbon dioxide and hydrogen, which are reducing agents. These gases tend to produce a digging arc that provides deep penetration. The weld deposit is somewhat rough, and the spatter is at a higher level than other electrodes. It does provide extremely good mechanical properties, particularly after aging. This is one of the earliest types of electrodes developed, and is widely used for cross country pipe lines using the downhill welding technique. It is normally used with direct current with the electrode positive (reverse polarity).
(2) Cellulose-potassium (EXX11). This electrode is very similar to the cellulose-sodium electrode, except more potassium is used than sodium. This provides ionization of the arc and makes the electrode suitable for welding with alternating current. The arc action, the penetration, and the weld results are very similar. In both E6010 and E6011 electrodes, small amounts of iron powder may be added. This assists in arc stabilization and will slightly increase the deposition rate.
(3) Rutile-sodium (EXX12). When rutile or titanium dioxide content is relatively high with respect to the other components, the electrode will be especially appealing to the welder. Electrodes with this coating have a quiet arc, an easily controlled slag, and a low level of spatter. The weld deposit will have a smooth surface and the penetration will be less than with the cellulose electrode. The weld metal properties will be slightly lower than the cellulosic types. This type of electrode provides a fairly high rate of deposition. It has a relatively low arc voltage, and can be used with alternating current or with direct current with electrode negative (straight polarity).
(4) Rutile-potassium (EXX13). This electrode coating is very similar to the rutile-sodium type, except that potassium is used to provide for arc ionization. This makes it more suitable for welding with alternating current. It can also be used with direct current with either polarity. It produces a very quiet, smooth running arc.
(5) Rutile-iron powder (EXXX4). This coating is very similar to the rutile coatings mentioned above, except that iron powder is added. If iron content is 25 to 40 percent, the electrode is EXX14. If iron content is 50 percent or more, the electrode is EXX24. With the lower percentage of iron powder, the electrode can be used in all positions. With the higher percentage of iron paler, it can only be used in the flat position or for making horizontal fillet welds. In both cases, the deposition rate is increased, based on the amount of iron powder in the coating.
(6) Low hydrogen-sodium (EXXX5). Coatings that contain a high proportion of calcium carbonate or calcium fluoride are called low hydrogen, lime ferritic, or basic type electrodes. In this class of coating, cellulose, clays, asbestos, and other minerals that contain combined water are not used. This is to ensure the lowest possible hydrogen content in the arc atmosphere. These electrode coatings are baked at a higher temperature. The low hydrogen electrode family has superior weld metal properties. They provide the highest ductility of any of the deposits. These electrodes have a medium arc with medium or moderate penetration. They have a medium speed of deposition, but require special welding techniques for best results. Low hydrogen electrodes must be stored under controlled conditions. This type is normally used with direct current with electrode positive (reverse polarity).
(7) Low hydrogen-potassium (EXXX6). This type of coating is similar to the low hydrogen-sodium, except for the substitution of potassium for sodium to provide arc ionization. This electrode is used with alternating current and can be used with direct current, electrode positive (reverse polarity). The arc action is smother, but the penetration of the two electrodes is similar.
(8) Low hydrogen-potassium (EXXX6). The coatings in this class of electrodes are similar to the low-hydrogen type mentioned above. However, iron powder is added to the electrode, and if the content is higher than 35 to 40 percent, the electrode is classified as an EXX18.
(9) Low hydrogen-iron powder (EXX28). This electrode is similar to the EXX18, but has 50 percent or more iron powder in the coating. It is usable only when welding in the flat position or for making horizontal fillet welds. The deposition rate is higher than EXX18. Low hydrogen coatings are used for all of the higher-alloy electrodes. By additions of specific metals in the coatings, these electrodes become the alloy types where suffix letters are used to indicate weld metal compositions. Electrodes for welding stainless steel are also the low-hydrogen type.
(10) Iron oxide-sodium (EXX20). Coatings with high iron oxide content produce a weld deposit with a large amount of slag. This can be difficult to control. This coating type produces high-speed deposition, and provides medium penetration with low spatter level. The resulting weld has a very smooth finish. The electrode is usable only with flat position welding and for making horizontal fillet welds. The electrode can be used with alternating current or direct current with either polarity.
(11) Iron-oxide-iron power (EXX27). This type of electrode is very similar to the iron oxide-sodium type, except it contains 50 percent or more iron power. The increased amount of iron power greatly increases the deposition rate. It may be used with alternating direct current of either polarity.
(12) There are many types of coatings other than those mentioned here, most of which are usually combinations of these types but for special applications such as hard surfacing, cast iron welding, and for nonferrous metals.
c. Classification and Storage of Electrodes. Refer to paragraph 5-25 for classification and storage of electrodes.
d. Deposition Rates. The different types of electrodes have different deposition rates due to the composition of the coating. The electrodes containing iron power in the coating have the highest deposition rates. In the United States, the percentage of iron power in a coating is in the 10 to 50 percent range. This is based on the amount of iron power in the coating versus the coating weight. This is shown in the formula:
These percentages are related to the requirements of the American Welding Society (AWS) specifications. The European method of specifying iron power is based on the weight of deposited weld metal versus the weight of the bare core wire consumed. This is shown as follows:
Thus, if the weight of the deposit were double the weight of the core wire, it would indicate a 200 percent deposition efficiency, even though the amount of the iron power in the coating represented only half of the total deposit. The 30 percent iron power formula used in the United States would produce a 100 to 110 percent deposition efficiency using the European formula. The 50 percent iron power electrode figured on United States standards would produce an efficiency of approximately 150 percent using the European formula.
e. Light Coated Electrodes.
(1) Light coated electrodes have a definite composition. A light coating has been applied on the surface by washing, dipping, brushing, spraying, tumbling, or wiping. The coatings improve the characteristics of the arc stream. They are listed under the E45 series in the electrode identification system, refer to paragraph 5-25.
(2) The coating generally serves the functions described below:
(a) It dissolves or reduces impurities such as oxides, sulfur, and phosphorus.
(b) It changes the surface tension of the molten metal so that the globules of metal leaving the end of the electrode are smaller and more frequent. This helps make flow of molten metal more uniform.
(c) It increases the arc stability by introducing materials readily ionized (i.e., changed into small particles with an electric charge) into the arc stream.
(3) Some of the light coatings may produce a slag. The slag is quite thin and does not act in the same manner as the shielded arc electrode type slag.
f. Shielded Arc or Heavy Coated Electrodes. Shielded arc or heavy coated electrodes have a definite composition on which a coating has been applied by dipping or extrusion. The electrodes are manufactured in three general types: those with cellulose coatings; those with mineral coatings; and those whose coatings are combinations of mineral and cellulose. The cellulose coatings are composed of soluble cotton or other forms of cellulose with small amounts of potassium, sodium, or titanium, and in some cases added minerals. The mineral coatings consist of sodium silicate, metallic oxides clay, and other inorganic substances or combinations thereof. Cellulose coated electrodes protect the molten metal with a gaseous zone around the arc as well as the weld zone. The mineral coated electrode forms a slag deposit. The shielded arc or heavy coated electrodes are used for welding steels, cast iron, and hard surfacing.
g. Functions of Shielded Arc or Heavy Coated Electrodes.
(1) These electrodes produce a reducing gas shield around the arc. This prevents atmospheric oxygen or nitrogen from contaminating the weld metal. The oxygen readily combines with the molten metal, removing alloying elements and causing porosity. Nitrogen causes brittleness, low ductility, and in Some cases low strength and poor resistance to corrosion.
(2) They reduce impurities such as oxides, sulfur, and phosphorus so that these impurities will not impair the weld deposit.
(3) They provide substances to the arc which increase its stability. This eliminates wide fluctuations in the voltage so that the arc can be maintained without excessive spattering.
(4) By reducing the attractive force between the molten metal and the end of the electrodes, or by reducing the surface tension of the molten metal, the vaporized and melted coating causes the molten metal at the end of the electrode to break up into fine, small particles.
(5) The coatings contain silicates which will form a slag over the molten weld and base metal. Since the slag solidifies at a relatively slow rate, it holds the heat and allows the underlying metal to cool and solidify slowly. This slow solidification of the metal eliminates the entrapment of gases within the weld and permits solid impurities to float to the surface. Slow cooling also has an annealing effect on the weld deposit.
(6) The physical characteristics of the weld deposit are modified by incorporating alloying materials in the electrode coating. The fluxing action of the slag will also produce weld metal of better quality and permit welding at higher speeds.
h. Direct Current Arc Welding Electrodes.
(1) The manufacturer's recommendations should be followed when a specific type of electrode is being used. In general, direct current shielded arc electrodes are designed either for reverse polarity (electrode positive) or for straight polarity (electrode negative), or both. Many, but not all, of the direct current electrodes can be used with alternating current. Direct current is preferred for many types of covered nonferrous, bare and alloy steel electrodes. Recommendations from the manufacturer also include the type of base metal for which given electrodes are suitable, corrections for poor fit-ups, and other specific conditions.
(2) In most cases, reverse polarity electrodes will provide more penetration than straight polarity electrodes. Good penetration can be obtained from either type with proper welding conditions and arc manipulation.
i. Alternating Current Arc Welding Electrodes.
(1) Coated electrodes which can be used with either direct or alternating current are available. Alternating current is more desirable while welding in restricted areas or when using the high currents required for thick sections because it reduces arc blow. Arc blow causes blowholes, slag inclusions, and lack of fusion in the weld.
(2) Alternating current is used in atomic hydrogen welding and in those carbon arc processes that require the use of two carbon electrodes. It permits a uniform rate of welding and electrode consumption ion. In carbon-arc processes where one carbon electrode is used, direct current straight polarity is recommended, because the electrode will be consumed at a lower rate.
j. Electrode Defects and Their Effect.
(1) If certain elements or oxides are present in electrode coatings, the arc stability will be affected. In bare electrodes, the composition and uniformity of the wire is an important factor in the control of arc stability. Thin or heavy coatings on the electrodes will not completely remove the effects of defective wire.
(2) Aluminum or aluminum oxide (even when present in quantities not exceeding 0.01 percent), silicon, silicon dioxide, and iron sulfate cause the arc to be unstable. Iron oxide, manganese oxide, calcium oxide, and iron sulfide tend to stabilize the arc.
(3) When phosphorus or sulfur are present in the electrode in excess of 0.04 percent, they will impair the weld metal. They are transferred from the electrode to the molten metal with very little loss. Phosphorus causes grain growth, brittleness, and "cold shortness" (i.e., brittle when below red heat) in the weld. These defects increase in magnitude as the carbon content of the steel increases. Sulfur acts as a slag, breaks up the soundness of the weld metal, and causes "hot shortness" (i.e., brittle when above red heat). Sulfur is particularly harmful to bare low carbon steel electrodes with a low manganese content. Manganese promotes the formation of sound welds.
(4) If the heat treatment given the wire core of an electrode is not uniform, the electrode will produce welds inferior to those produced with an electrode of the same composition that has been properly heat treated.
8-2. SOLID ELECTRODE WIRES
a. General. Bare or solid wire electrodes are made of wire compositions required for specific applications, and have no coatings other than those required in wire drawing. These wire drawing coatings have a slight stabilizing effect on the arc, but are otherwise of no consequence. Bare electrodes are used for welding manganese steels and for other purposes where a covered electrode is not required or is undesirable. A sketch of the transfer of metal across the arc of a bare electrode is shown in figure 8-1.
b. Solid steel electrode wires may not be bare. Many have a very thin copper coating on the wire. The copper coating improves the current pickup between contact tip and the electrode, aids drawing, and helps prevent rusting of the wire when it is exposed to the atmosphere. Solid electrode wires are also made of various stainless steels, aluminum alloys, nickel alloys, magnesium alloys, titanium alloys, copper alloys, and other metals.
c. When the wire is cut and straightened, it is called a welding rod, which is a form of filler metal used for welding or brazing and does not conduct the electrical current. If the wire is used in the electrical circuit, it is called a welding electrode, and is defined as a component of the welding circuit through which current is conducted. A bare electrode is normally a wire; however, it can take other forms.
d. Several different systems are used to identify the classification of a particular electrode or welding rod. In all cases a prefix letter is used.
(1) Prefix R. Indicates a welding rod.
(2) Prefix E. Indicates a welding electrode.
(3) Prefix RB. Indicates use as either a welding rod or for brazing filler metal.
(4) Prefix ER. Indicates wither an electrode or welding rod.
e. The system for identifying bare carbon steel electrodes and rods for gas shielded arc welding is as follows:
(1) ER indicates an electrode or welding rod.
(2) 70 indicates the required minimum as-welded tensile strength in thousands of pounds per square inch (psi).
(3) S indicates solid electrode or rod.
(4) C indicates composite metal cored or stranded electrode or rod.
(5) 1 suffix number indicates a particular analysis and usability factor.
Table 8-1. Mild Steel Electrode Wire Composition for Submerged Arc Welding
f. Submerged Arc Electrodes. The system for identifying solid bare carbon steel for submerged arc is as follows:
(1) The prefix letter E is used to indicate an electrode. This is followed by a letter which indicates the level of manganese, i.e., L for low, M for medium, and H for high manganese. This is followed by a number which is the average amount of carbon in points or hundredths of a percent. The composition of some of these wires is almost identical with some of the wires in the gas metal arc welding specification.
(2) The electrode wires used for submerged arc welding are given in American Welding Society specification, "Bare Mild Steel Electrodes and Fluxes for Submerged Arc Welding." This specification provides both the wire composition and the weld deposit chemistry based on the flux used. The specification does give composition of the electrode wires. This information is given in table 8-1. When these electrodes are used with specific submerged arc fluxes and welded with proper procedures, the deposited weld metal will meet mechanical properties required by the specification.
(3) In the case of the filler reds used for oxyfuel gas welding, the prefix letter is R, followed by a G indicating that the rod is used expressly for gas welding. These letters are followed by two digits which will be 45, 60, or 65. These designate the approximate tensile strength in 1000 psi (6895 kPa).
(4) In the case of nonferrous filler metals, the prefix E, R, or RB is used, followed by the chemical symbol of the principal metals in the wire. The initials for one or two elements will follow. If there is more than one alloy containing the same elements, a suffix letter or number may be added.
(5) The American Welding Society's specifications are most widely used for specifying bare welding rod and electrode wires. There are also military specifications such as the MIL-E or -R types and federal specifications, normally the QQ-R type and AMS specifications. The particular specification involved should be used for specifying filler metals.
g. The most important aspect of solid electrode wires and rods in their composition, which is given by the specification. The specifications provide the limits of composition for the different wires and mechanical property requirements.
h. Occasionally, on copper-plated solid wires, the copper may flake off in the feed roll mechanism and create problems. It may plug liners, or contact tips. A light copper coating is desirable. The electrode wire surface should be reasonably free of dirt and drawing compounds. This can be checked by using a white cleaning tissue and pulling a length of wire through it. Too much dirt will clog the liners, reduce current pickup in the tip, and may create erratic welding operation.
i. Temper or strength of the wire can be checked in a testing machine. Wire of a higher strength will feed through guns and cables better. The minimum tensile strength recommended by the specification is 140,000 psi (965,300 kPa).
j. The continuous electrode wire is available in many different packages. They range from extremely small spools that are used on spool guns, through medium-size spools for fine-wire gas metal arc welding. Coils of electrode wire are available which can be placed on reels that are a part of the welding equipment. There are also extremely large reels weighing many hundreds of pounds. The electrode wire is also available in drums or payoff packs where the wire is laid in the round container and pulled from the container by an automatic wire feeder.
8-3. FLUX-CORED OR TUBULAR ELECTRODES
a. General. The flux-cored arc welding process is made possible by the design of the electrode. This inside-outside electrode consists of a metal sheath surrounding a core of fluxing and alloying compounds. The compounds contained in the electrode perform essentially the same functions as the coating on a covered electrode, i.e., deoxidizers, slag formers, arc stabilizers, alloying elements, and may provide shielding gas. There are three reasons why cored wires are developed to supplement solid electrode wires of the same or similar analysis.
(1) There is an economic advantage. Solid wires are drawn from steel billets of the specified analyses. These billets are not readily available and are expensive. A single billet might also provide more solid electrode wire than needed. In the case of cored wires, the special alloying elements are introduced in the core material to provide the proper deposit analysis.
(2) Tubular wire production method provides versatility of composition and is not limited to the analysis of available steel billets.
(3) Tubular electrode wires are easier for the welder to use than solid wires of the same deposit analysis, especially for welding pipe in the fixed position.
b. Flux-Cored Electrode Design. The sheath or steel portion of the flux-cored wire comprises 75 to 90 percent of the weight of the electrode, and the core material represents 10 to 25 percent of the weight of the electrode.
For a covered electrode, the steel represents 75 percent of the weight and the flux 25 percent. This is shown in more detail below:
|
Flux Cored Electrode Wire |
Covered Electrode |
||||
|
|
|
||||
| By area | Flux | 25% | By area | Flux | 55% |
| steel | 75% | steel | 45% | ||
| By weight | Flux | 15% | By weight | Flux | 24% |
| steel | 85% | steel | 76% | ||
More flux is used on covered electrodes than in a flux-cored wire to do the same job. This is because the covered electrode coating contains binders to keep the coating intact and also contains agents to allow the coating to be extruded.
c. Self-Shielding Flux-Cored Electrodes. The self-shielding type flux-cored electrode wires include additional gas forming elements in the core. These are necessary to prohibit the oxygen and nitrogen of the air from contacting the metal transferring across the arc and the molten weld puddle. Self-shielding electrodes also include extra deoxidizing and denigrating elements to compensate for oxygen and nitrogen which may contact the molten metal. Self-shielding electrodes are usually more voltage-sensitive and require electrical stickout for smooth operation. The properties of the weld metal deposited by the self-shielding wires are sometimes inferior to those produced by the externally shielded electrode wires because of the extra amount of deoxidizers included. It is possible for these elements to build up in multipass welds, lower the ductility, and reduce the impact values of the deposit. Some codes prohibit the use of self-shielding wires on steels with yield strength exceeding 42,000 psi (289,590 kPa). Other codes prohibit the self-shielding wires from being used on dynamically loaded structures.
d. Metal Transfer. Metal transfer from consumable electrodes across an arc has been classified into three general modes. These are spray transfer, globular transfer, and short circuiting transfer. The metal transfer of flux-cored electrodes resembles a fine globular transfer. On cored electrodes in a carbon dioxide shielding atmosphere, the molten droplets build up around the outer sheath of the electrode. The core material appears to transfer independently to the surface of the weld puddle. At low currents, the droplets tend to be larger than when the current density is increased. Transfer is more frequent with smaller drops when the current is increased. The larger droplets at the lower currents cause a certain amount of splashing action when they enter the weld puddle. This action decreases with the smaller droplet size. This explains why there is less visible spatter, the arc appears smoother to the welder, and the deposition efficiency is higher when the electrode is used at high current rather than at the low end of its current range.
e. Mild Steel Electrodes. Carbon steel electrodes are classified by the American Welding Society specification, "Carbon Steel Electrodes for Flux-cored-Arc Welding". This specification includes electrodes having no appreciable alloy content for welding mild and low alloy steels. The system for identifying flux-cored electrodes follows the same pattern as electrodes for gas metal arc welding, but is specific for tubular electrodes. For example, in E70T-1, the E indicates an electrode; 70 indicates the required minimum as-welded tensile strength in thousands of pounds per square inch (psi); T indicates tubular, fabricated, or flux-cored electrode; and 1 indicates the chemistry of the deposited weld metal, gas type, and usability factor.
f. Classification of Flux-Cored Electrodes.
(1) E60T-7 electrode classification. Electrodes of this classification are used without externally applied gas shielding and may be used for single-and multiple-pass applications in the flat and horizontal positions. Due to low penetration and to other properties, the weld deposits have a low sensitivity to cracking.
(2) E60T-8 electrode classifications. Electrodes of this classification are used without externally applied gas shielding and may be used for single-and multiple-pass applications in the flat and horizontal positions. Due to low penetration and to other properties, the weld deposits have a low sensitivity to cracking.
(3) E70T-1 electrode classification. Electrodes of this classification are designed to be used with carbon dioxide shielding gas for single-and multiple-pass welding in the flat position and for horizontal fillets. A quiet arc, high-deposition rate, low spatter loss, flat-to-slightly convex bead configuration, and easily controlled and removed slag are characteristics of this class.
(4) E70T-2 electrode classification. Electrodes of this classification are used with carbon dioxide shielding gas and are designed primarily for single-pass welding in the flat position and for horizontal fillets. However, multiple-pass welds can be made when the weld beads are heavy and an appreciable amount of mixture of the base and filler metals occurs.
(5) E70T-3 electrode classification. Electrodes of this classification are used without externally applied gas shielding and are intended primarily for depositing single-pass, high-speed welds in the flat and horizontal positions on light plate and gauge thickness base metals. They should not be used on heavy sections or for multiple-pass applications.
(6) E70T-4 electrode classification. Electrodes of this classification are used without externally applied gas shielding and may be used for single-and multiple-pass applications in the flat and horizontal positions. Due to low penetration, and to other properties, the weld deposits have a low sensitivity to cracking.
(7) E70T-5 electrode classification. This classification covers electrodes primarily designed for flat fillet or groove welds with or without externally applied shielding gas. Welds made using-carbon dioxide shielding gas have better quality than those made with no shielding gas. These electrodes have a globular transfer, low penetration, slightly convex bead configuration, and a thin, easily removed slag.
(8) E70T-6 electrode classification. Electrodes of this classification are similar to those of the E70T-5 classification, but are designed for use without an externally applied shielding gas.
(9) E70T-G electrode classification. This classification includes those composite electrodes that are not included in the preceding classes. They may be used with or without gas shielding and may be used for multiple-pass work or may be limited to single-pass applications. The E70T-G electrodes are not required to meet chemical, radiographic, bend test, or impact requirements; however, they are required to meet tension test requirements. Welding current type is not specified.
g. The flux-cored electrode wires are considered to be low hydrogen, since the materials used in the core do not contain hydrogen. However, some of these materials are hydroscopic and thus tend to absorb moisture when exposed to a high-humidity atmosphere. Electrode wires are packaged in special containers to prevent this. These electrode wires must be stored in a dry room.
h. Stainless Steel Tubular Wires. Flux-cored tubular electrode wires are available which deposit stainless steel weld metal corresponding to the A.I.S.I. compositions. These electrodes are covered by the A.W.S specification, "Flux-Cored Corrosion Resisting Chromium and Chromium-Nickel Steel Electrodes." These electrodes are identified by the prefix E followed by the standard A.I.S.I. code number. This is followed by the letter T indicating a tubular electrode. Following this and a dash are four-possible suffixes as follows:
(1) -1 indicates the use of C02 (carbon dioxide) gas for shielding and DCEP.
(2) -2 indicates the use of argon plus 2 percent oxygen for shielding and DCEP.
(3) -3 indicates no external gas shielding and DCEP.
(4) -G indicates that gas shielding and polarity are not specified.
Tubular or flux-cored electrode wires are also used for surfacing and submerged arc welding applications.
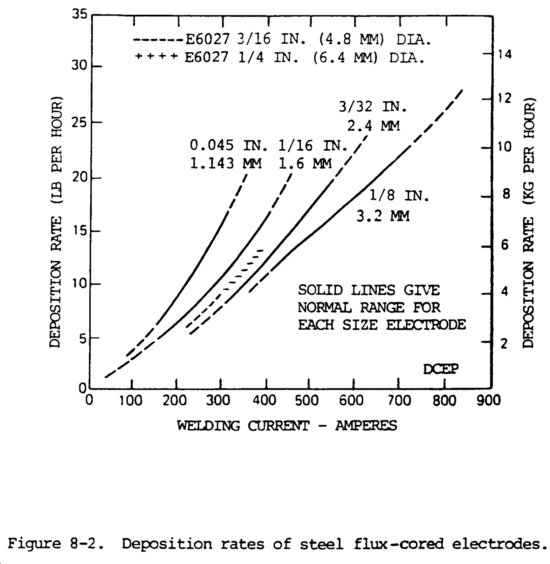
i. Deposition Rates and Weld Quality. The deposition rates for flux-cored electrodes are shown in figure 8-2. These curves show deposition rates when welding with mild and low-alloy steel using direct current electrode positive. Two type of of covered electrodes are shown for comparison. Deposition rates of the smaller size flux-cored wires exceed that of the covered electrodes. The metal utilization of the flux-cored electrode is higher. Flux-cored electrodes have a much broader current range than covered electrodes, which increases the flexibility of the process. The quality of the deposited weld metal produced by the flux-cored arc welding process depends primarily on the flux-cored electrode wire that is used. It can be expected that the deposited weld metal will match or exceed the properties shown for the electrode used. This assures the proper matching of base metal, flux-cored electrode type and shielding gas. Quality depends on the efficiency of the gas shielding envelope, on the joint detail, on the cleanliness of the joint, and on the skill of the welder. The quality level of of weld metal deposited by the self-shielding type electrode wires is usually lower than that produced by electrodes that utilize external gas shielding.
Section II. OTHER FILLER METALS
8-4. GENERAL
There are other filler metals and special items normally used in making welds. These include the nonconsumable electrodes (tungsten and carbon), and other materials, including backing tapes, backing devices, flux additives, solders, and brazing alloys. Another type of material consumed in making a weld are the consumable rings used for root pass welding of pipe. There are also ferrules used for stud welding and the guide tubes in the consumable guide electroslag welding method. Other filler materials are solders and brazing alloys.
8-5. NONCONSUMABLE ELECTRODES
a. Types of Nonconsumable Electrodes. There are two types of nonconsumable electrodes. The carbon electrode is a non-filler metal electrode used in arc welding or cutting, consisting of a carbon graphite rod which may or may not be coated with copper or other coatings. The second nonconsumable electrode is the tungsten electrode, defined as a non-filler metal electrode used in arc welding or cutting, made principally of tungsten.
b. Carbon Electrodes. The American Welding Society does not provide specification for carbon electrodes but there is a military specification, no. MIL-E-17777C, entitled, "Electrodes Cutting and Welding Carbon-Graphite Uncoated and Copper Coated". This specification provides a classification system based on three grades: plain, uncoated, and copper coated. It provides diameter information, length information, and requirements for size tolerances, quality assurance, sampling, and various tests. Applications include carbon arc welding, twin carbon arc welding, carbon cutting, and air carbon arc cutting and gouging.
c. Tungsten Electrodes.
(1) Nonconsumable electrodes for gas types: pure tungsten, tungsten containing tungsten arc (TIG) welding are of four 1.0 percent thorium, tungsten containing 2.0 percent thorium, and tungsten containing 0.3 to 0.5 percent zirconium. They are also used for plasma-arc and atomic hydrogen arc welding.
(2) Tungsten electrodes can be identified by painted end marks:
(a) Green - pure tungsten.
(b) Yellow - 1.0 percent thorium.
(c) Red - 2.0 percent thorium.
(d) Brown - 0.3 to 0.5 percent zirconium.
(3) Pure tungsten (99. 5 percent tungsten) electrodes are generally used on less critical welding operations than the tungstens which are alloyed. This type of electrode has a relatively low current carrying capacity and a low resistance to contamination.
(4) Thoriated tungsten electrodes (1.0 or 2.0 percent thorium) are superior to pure tungsten electrodes because of their higher electron output, better arc starting and arc stability, high current-carrying capacity, longer life, and greater resistance to contamination.
(5) Tungsten electrodes containing 0.3 to 0.5 percent zirconium generally fall between pure tungsten electrodes and thoriated tungsten electrodes in terms of performance. There is, however, some indication of better performance in certain types of welding using ac power.
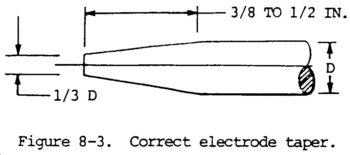
(6) Finer arc control can be obtained if the tungsten alloyed electrode is ground to a point (fig. 8-3). When electrodes are not grounded, they must be operated at maximum current density to obtain reasonable arc stability. Tungsten electrode points are difficult to maintain if standard direct current equipment is used as a power source and touch--starting arc is standard practice. Maintenance of electrode shape and the reduction of tungsten inclusions in the weld can best be ground by superimposing a high-frequency current on the regular welding current. Tungsten electrodes alloyed with thorium retain their shape longer when touch-starting is used. Unless high frequency alternating current is available, touch-starting must be used with thorium electrodes.
(7) The electrode extension beyond the gas cup is determined by the type of joint being welded. For example, an extension beyond the gas cup of 1/8 in. (0.32 cm) might be used for butt joints in light gauge material, while an extension of approximately 1/4 to 1/2 in. (0.64 to 1.27 cm) might be necessary on some fillet welds. The tungsten electrode or torch should be inclined slightly and the filler metal added carefully to avoid contact with the tungsten to prevent contamination of the electrode. If contamination does occur, the electrode must be removed, reground, and replaced in the torch.
d. Backing Materials. Backing materials are being used more frequently for welding. Special tapes exist, some of which include small amounts of flux, which can be used for backing the roots of joints. There are also different composite backing materials, for one-side welding. Consumable rings are used for making butt welds in pipe and tubing. These are rings made of metal that are tack welded in the root of the weld joint and are fused into the joint by the gas tungsten arc. There are three basic types of rings called consumable inert rings which are available in different analyses of metal based on normal specifications.
8-6. SUBMERGED ARC FLUX ADDITIVES
Specially processed metal powder is sometimes added to the flux used for the submerged arc welding process. Additives are provided to increase productivity or enrich the alloy composition of the deposited weld metal. In both cases, the additives are of a proprietary nature and are described by their manufacturers, indicating the benefit derived by using the particular additive. Since there are no specifications covering these types of materials, the manufacturer's information must be used.
8-7. SOLDERING
a. General. Soldering is the process of using fusible alloys for joining metals. The kind of solder used depends on the metals being joined. Hard solders are called spelter, and hard soldering is called silver solder brazing. This process gives greater strength and will withstand more heat than soft solder. Soft soldering is used for joining most common metals with an alloy that melts at a temperature below that of the base metal, and always below 800°F (427°C). In many respects, this is similar to brazing, in that the base is not melted, but merely tinned on the surface by the solder filler metal. For its strength, the soldered joint depends on the penetration of the solder into the pores of the base metal and. the formation of a base metal-alloy solder.
b. Solders of the tin-lead alloy system constitute the largest portion of all solders in use. They are used for joining most metals and have good corrosion resistance to most materials. Most cleaning and soldering processes may be used with the tin-lead solders. Other solders are: tin-antimony; tin-antimony-lead; tin-silver; tin-lead-silver; tin-zinc; cadmium-silver; cadmium-zinc; zinc-aluminum; bismuth (fusible) solder; and indium solders. These are described below. Fluxes of all types can also be used; the choice depends on the base metal to be joined.
(1) Tin-antinmony solder. The 95 percent tin-5 percent antimony solder provides a narrow melting range at a temperature higher than the tin-lead eutectic. the solder is used many plumbing, refrigeration, and air conditioning applications because of its good creep strength.
(2) Tin-antimony-lead solders. Antimony may be added to a tin-lead solder as a substitute for some of the tin. The addition of antimony up to 6 percent of the tin content increases the mechanical properties of the solder with only slight impairment to the soldering characteristics. All standard methods of cleaning, fluxing, and heating may be used.
(3) Tin-silver and tin-lead-silver solders. The 96 percent tin-4 percent silver solder is free of lead and is often used to join stainless steel for food handling equipment. It has good shape and creep strengths, and excellent flow characteristics. The 62 percent tin-38 percent lead-2 percent silver solder is used when soldering silver-coated surfaces for electronic applications. The silver addition retards the dissolution of the silver coating during the soldering operation. The addition of silver also increases creep strength. The high lead solders containing tin and silver provide higher temperature solders or many applications. They exhibit good tensile, shear, and creep strengths and are recommended for cryogenic applications. Because of their high melting range, only inorganic fluxes are recommended for use with these solders.
(4) Tin-zinc solders. A large number of tin-zinc solders have come into use for joining aluminum. Galvanic corrosion of soldered joints in aluminum is minimized if the metals in the joint are close to each other in the electrochemical series. Alloys containing 70 to 80 percent tin with the balance zinc are recommended for soldering aluminum. The addition of 1 to 2 percent aluminum, or an increase of the zinc content to as high as 40 percent, improves corrosion resistance. However, the liquidus temperature rises correspondingly, and these solders are therefore more difficult to apply. The 91/9 and 60/40 tin-zinc solders may be used for high temperature applications (above 300°F (149°C)), while the 80/20 and the 70/30 tin-zinc solders are generally used to coat parts before soldering.
CAUTION
Cadmium fumes can be health hazards. Improper use of solders containing cadmium can be hazardous to personnel.
(5) Cadmium-silver solder. The 95 percent cadmium-5 percent silver solder is in applications where service temperatures will be higher than permissible with lower melting solders. At room temperature, butt joints in copper can be made to produce tensile strengths of 170 MPa (25,000 psi). At 425°F (218°C), a tensile strength of 18 MPa (2600 psi) can be obtained. Joining aluminum to itself or to other metals is possible with this solder. Improper use of solders containing cadmium may lead to health hazards. Therefore, care should be taken in their application, particularly with respect to fume inhalation.
(6) Cadmium-zinc solders. These solders are also useful for soldering aluminum. The cadmium-zinc solders develop joints with intermediate strength and corrosion resistance when used with the proper flux. The 40 percent cadmium-60 percent zinc solder has found considerable use in the soldering of aluminum lamp bases. Improper use of this solder may lead to health hazards, particularly with respect to fume inhalation.
(7) Zinc-aluminum solder. This solder is specifically for use on aluminum. It develops joints with high strength and good corrosion resistance. The solidus temperature is high, which limits its use to applications where soldering temperature is in excess of 700°F (371°C) can be tolerated. A major application is in dip soldering the return bends of aluminum air conditioner coils. Ultrasonic solder pots are employed without the use of flux. In manual operations, the heated aluminum surface is rubbed with the solder stick to promote wetting without a flux.
(8) Fusible alloys. Bismuth-containing solders, the fusible alloys, are useful for soldering operations where soldering temperatures helm 361°F (183°C) are required. The low melting temperature solders have applications in cases such as soldering heat treated surfaces where higher soldering temperatures would result in the softening of the part; soldering joints where adjacent material is very sensitive to temperature and would deteriorate at higher soldering temperatures; step soldering operations where a low soldering temperature is necessary to avoid destroying a nearby joint that has been made with a higher melting temperature solder; and on temperature-sensing devices, such as fire sprinkler systems, where the device is activated when the fusible alloy melts at relatively low temperature. Many of these solders, particularly those containing a high percentage of bismuth, are very difficult to use successfully in high-speed soldering operations. Particular attention must be paid to the cleanliness of metal surfaces. Strong, corrosive fluxes must be used to make satisfactory joints on uncoated surfaces of metals, such as copper or steel. If the surface can be plated for soldering with such metals as tin or tin-lead, noncorrosive rosin fluxes may be satisfactory; however, they are not effective below 350°F (177°C).
(9) Indium solders. These solders possess certain properties which make them valuable for some special applications. Their usefulness for any particular application should be checked with the supplier. A 50 percent indium-50 percent tin alloy adheres to glass readily and may be used for glass-to-metal and glass-to-glass soldering. The low vapor pressure of this alloy makes it useful for seals in vacuum systems. Iridium solders do not require special techniques during use. All of the soldering methods, fluxes, and techniques used with the tin-lead solders are applicable to iridium solders.
8-8. BRAZING ALLOYS
a. General.
(1) Brazing is similar to the soldering processes in that a filler rod with a melting point lower than that of the base metal, but stove 800°F (427°C) is used. A groove, fillet, plug, or slot weld is made and the filler metal is distributed by capillary attraction. In brazing, a nonferrous filler rod, strip, or wire is used for repairing or joining cast iron, malleable iron, wrought iron, steel, copper, nickel, and high melting point brasses and bronzes. Some of these brasses and bronzes, however, melt at a temperature so near to that of the filler rod that fusion welding rather than brazing is required.
(2) Besides a welding torch with a proper tip size, a filler metal of the required composition and a proper flux are important to the success of any brazing operation.
(3) The choice of the filler metal depends on the types of metals to be joined. Copper-silicon (silicon-bronze) rods are used for brazing copper and copper alloys. Copper-tin (phosphor-bronze) rods are used for brazing similar copper alloys and for brazing steel and cast iron. Other compositions are used for brazing specific metals.
(4) Fluxes are used to prevent oxidation of the filler metal and the base metal surface, and to promote the free flowing of the filler metal. They should be chemically active and fluid at the brazing temperature. After the joint members have been fitted and thoroughly cleaned, an even coating of flux should be brushed over the adjacent surfaces of the joint, taking care that no spots are left uncovered. The proper flux is a good temperate indicator for torch brazing because the joint should be heated until the flux remains fluid when the torch flame is momentarily removed.
b. Characteristics. For satisfactory use in brazing applications, brazing filler metals must possess the following properties:
(1) The ability to form brazed joints possessing suitable mechanical and physical properties for the intended service application.
(2) A melting point or melting range compatible with the base metals being joined and sufficient fluidity at brazing temperature to flow and distribute into properly prepared joints by capillary action.
(3) A composition of sufficient homogeneity and stability to minimize separation of constituents (liquation) under the brazing conditions encountered.
(4) The ability to wet the surfaces of the base metals being joined and form a strong, sound bond.
(5) Depending on the requirements, ability to produce or avoid base metal-filler metal interactions.
c. Filler Metal Selection. The following factors should be considered when selecting a brazing filler metal:
(1) Compatibility with base metal and joint design.
(2) Service requirements for the brazed assembly. Compositions should be selected to suit operating requirements, such as service temperature (high or cryogenic), thermal cycling, life expectancy, stress loading, corrosive conditions, radiation stability, and vacuum operation.
(3) Brazing temperature required. Low brazing temperatures are usually preferred to economize on heat energy; minimize heat effects on base metal (annealing, grain growth, warpage, etc.); minimize base metal-filler metal interaction; and increase the life of fixtures and other teals. High brazing temperatures are preferred in order to take advantage of a higher melting, but more economical, brazing filler metal; to combine annealing, stress relief, or heat treatment of the base metal with brazing; to permit subsequent processing at elevated temperatures; to promote base metal-filler metal interactions to increase the joint remelt temperature; or to promote removal of certain refractory oxides by vacuum or an atmosphere.
(4) Method of heating. Filler metals with narrow melting ranges (less than 50°F (28°C) between solidus and liquidus) can be used with any heating method, and the brazing filler metal may be preplaced in the joint area in the form of rings, washers, formed wires, shims, powder, or paste. Such alloys may also be manually or automatically face fed into the joint after the base metal is heated. Filler metals that tend to liquate should be used with heating methods that bring the joint to brazing temperature quickly, or allow the introduction of the brazing filler metal after the base metal reaches the brazing temperature.
d. Aluminum-Silicon Filler Metals. This group is used for joining aluminum and aluminum alloys. They are suited for furnace and dip brazing, while some types are also suited for torch brazing using lap joints rather than butt joints. Flux should be used in all cases and removed after brazing, except when vacuum brazing. Use brazing sheet or tubing that consists of a core of aluminum alloy and a coating of lower melting filler metal to supply aluminum filler metal. The coatings are aluminum-silicon alloys and may be applied to one or both sides of sheet. Brazing sheet or tubing is frequently used as one member of an assembly with the mating piece made of an unclad brazeable alloy. The coating on the brazing sheet or tubing melts at brazing temperature and flows by capillary attraction and gravity to fill the joints.
e. Magnesium Filler Metals. Because of its higher melting range, one magnesium filler metal (BMg-1) is used for joining AZ10A, KIA, and MIA magnesium alloys, while the other alloy (BMg-2a), with a lower melting range, is used for the AZ31B and ZE10A compositions. Both filler metals are suited for torch, dip, or furnace brazing processes. Heating must be closely controlled with both filler metals to prevent melting of the base metal.
f. Copper and Copper-Zinc Filler Metals. These brazing filler metals are used for joining various ferrous metals and nonferrous metals. They are commonly used for lap and butt joints with various brazing processes. However, the corrosion resistance of the copper-zinc alloy filler metals is generally inadequate for joining copper, silicon bronze, copper-nickel alloys, or stainless steel.
(1) The essentially pure copper brazing filler metals are used for joining ferrous metals, nickel base, and copper-nickel alloys. They are very free flowing and are often used in furnace brazing with a combusted gas, hydrogen, or dissociated ammonia atmosphere without flux. However, with metals that have components with difficult-to-reduce oxides (chromium, manganese, silicon, titanium, vanadium, and aluminum), a higher quality atmosphere or mineral flux may be required. copper filler metals are available in wrought and powder forms.
(2) Copper-zinc alloy filler metals are used on most common base metals. A mineral flux is commonly used with the filler metals.
(3) Copper-zinc filler metals are used on steel, copper, copper alloys, nickel and nickel base alloys, and stainless steel where corrosion resistance is not a requirement. They are used with the torch, furnace, and induction brazing processes. Fluxing is required, and a borax-boric acid flux is commonly used.
g. Copper-Phosphorus Filler Metals. These filler metals are primarily used for joining copper and copper alloys and have some limited use for joining silver, tungsten, and molybdenum. They should not be used on ferrous or nickel base alloys, or on copper-nickel alloys with more than 10 percent nickel. These filler metals are suited for all brazing processes and have self fluxing properties when used on copper. However, flux is recommended with all other metals, including copper alloys.
h. Silver Filler Metals.
(1) These filler metals are used for joining most ferrous and nonferrous metals, except aluminum and magnesium, with all methods of heating. They may be prep laced in the joint or fed into the joint area after heating. Fluxes are generally required, but fluxless brazing with filler metals free of cadmium and zinc can be done on most metals in an inert or reducing atmosphere (such as dry hydrogen, dry argon, vacuum, and combusted fuel gas).
CAUTION
Do not overheat filler metals containing cadmium. Cadmium oxide fumes are hazardous.
(2) The addition of cadmium to the silver-copper-zinc alloy system lowers the melting and flew temperatures of the filler metal. Cadmium also increases the fluidity and wetting action of the filler metal on a variety of base metals. Cadmium bearing filler metals should be used with caution. If they are improperly used and subjected to overheating, cadmium oxide frees can be generated. Cadmium oxide fumes are a health hazard, and excessive inhalation of these fumes must be avoided.
(3) Of the elements that are commonly used to lower the melting and flow temperatures of copper-silver alloys, zinc is by far the most helpful wetting agent when joining alloys based on iron, cobalt, or nickel. Alone or in combination with cadmium or tin, zinc produces alloys that wet the iron group metals but do not alloy with them to any appreciable depth.
(4) Tin has a low vapor pressure at normal brazing temperatures. It is used in silver brazing filler metals in place of zinc or cadmium when volatile constituents are objectionable, such as when brazing is done without flux in atmosphere or vacuum furnaces, or when the brazed assemblies will be used in high vacuum at elevated temperatures. Tin additions to silver-copper alloys produce filler metals with wide melting ranges. Alloys containing zinc wet ferrous metals more effectively than those containing tin, and where zinc is tolerable, it is preferred to tin.
(5) Stellites, cemented carbides, and other molybdenum and tungsten rich refractory alloys are difficult to wet with the alloys previously mentioned. Manganese, nickel, and infrequently, cobalt, are often added as wetting agents in brazing filler metals for joining these materials. An important characteristic of silver brazing filler metals containing small additions of nickel is improved resistance to corrosion under certain conditions. They are particularly recommended where joints in stainless steel are to be exposed to salt water corrosion.
(6) When stainless steels and other alloys that form refractory oxides are to be brazed in reducing or inert atmospheres without flux, silver brazing filler metals containing lithium as the wetting agent are quite effective. Lithium is capable of reducing the adherent oxides on the base metal. The resultant lithium oxide is readily displaced by the brazing alloy. Lithium bearing alloys are advantageously used in very pure dry hydrogen or inert atmospheres.
i. Gold Filler Metals. These filler metals are used for joining parts in electron tube assemblies where volatile components are undesirable; and the brazing of iron, nickel, and cobalt base metals where resistance to oxidation or corrosion is required. Because of their low rate of interaction with the base metal, they are commonly used on thin sections, usually with induction, furnace, or resistance heating in a reducing atmosphere or in vacuum without flux. For certain applications, a borax-boric acid flux may be used.
j. Nickel Filler Metals.
(l) These brazing filler metals are generally used for their corrosion resistance and heat resistant properties up to 1800°F (982°C) continuous service, and 2200°F (1204°C) short time service, depending on the specific filler metals and operating environment. They are generally used on 300 and 400 series stainless steels and nickel and cobalt base alloys. Other base metals such as carbon steel, low alloy steels, and copper are also brazed when specific properties are desired. The filler metals also exhibit satisfactory room temperature and cryogenic temperature properties down to the liquid point of helium. The filler metals are normally applied as powders, pastes, or in the form of sheet or rod with plastic binders.
(2) The phosphorus containing filler metals exhibit the lowest ductility because of the presence of nickel phosphides. The boron containing filler metals should not be used for brazing thin sections because of their erosive action. The quantity of filler metal and time at brazing temperatures should be controlled because of the high solubility of some base metals in these filler metals.
k. Cobalt Filler Metal. This filler metal is generally used for its high temperature properties and its compatibility with cobalt base metals. For optimum results, brazing should be performed in a high quality atmosphere. Special high temperature fluxes are available.
1. Filler Metals for Refractory Metals.
(1) Brazing is an attractive means for fabricating many assemblies of refractory metals, in particular those involving thin sections. The use of brazing to join these materials is somewhat restricted by the lack of filler metals specifically designed for brazing them. Although several references to brazing are present, the reported filler metals that are suitable for applications involving both high temperature and high corrosion are very limited.
(2) Low melting filler metals, such as silver-copper-zinc, copper-phosphorus, and copper, are used to join tungsten for electrical contact applications. These filler metals are limited in their applications, however, because they cannot operate at very high temperatures. The use of higher melting metals, such as tantalum and columbium, is warranted in those cases. Nickel base and precious-metal base filler metals may be used for joining tungsten.
(3) A wide variety of brazing filler metals may be used to join molybdenum. The brazing temperature range is the same as that for tungsten. Each filler metal should be evaluated for its particular applicability. The service temperature requirement in many cases dictates the brazing filler metal selection. However, consideration must -be given to the effect of brazing temperature on the base metal properties, specifically recrystallization. When brazing above the recrystallization temperature, time should be kept as short as possible. When high temperature service is not required, copper and silver base filler metals may be used. For electronic parts and other nonstructural applications requiring higher temperatures, gold-copper, gold-nickel, and copper-nickel filler metals can be used. Higher melting metals and alloys may be used as brazing filler metals at still higher temperatures.
(4) Copper-gold alloys containing less than 40 percent gold can also be used as filler metals, but gold content between 46 and 90 percent tends to form age hardening compounds which are brittle. Although silver base filler metals have been used to join tantalum and columbium, they are not recommended because of a tendency to embrittle the base metals.
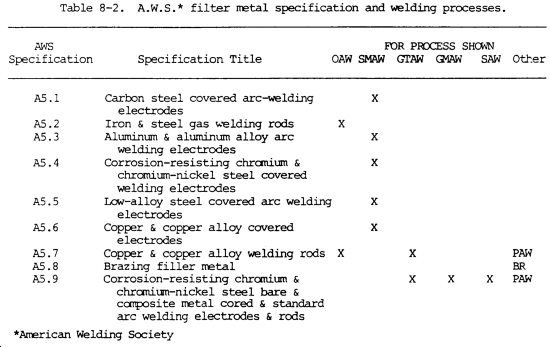
m. Filler metal specifications and welding processes are shown in table 8-2.