CHAPTER 7
METALS IDENTIFICATION
Section I. CHARACTERISTICS
7-1. GENERAL
Most of the metals and alloys used in Army materiel can be welded by one or more of the processes described in this manual. This section describes the characteristics of metals and their alloys, with particular reference to their significance in welding operations.
7-2. PROPERTIES OF METALS
a. Definitions. All metals fall within two categories, ferrous or nonferrous.
(1) Ferrous metals are metals that contain iron. Ferrous metals appear in the form of cast iron, carbon steel, and tool steel. The various alloys of iron, after undergoing certain processes, are pig iron, gray cast iron, white iron, white cast iron, malleable cast iron, wrought iron, alloy steel, and carbon steel. All these types of iron are mixtures of iron and carbon, manganese, sulfur, silicon, and phosphorous. Other elements are also present, but in amounts that do not appreciably affect the characteristics of the metal.
(2) Nonferrous metals are those which do not contain iron. Aluminum, copper, magnesium, and titanium alloys are among those metals which belong to this group.
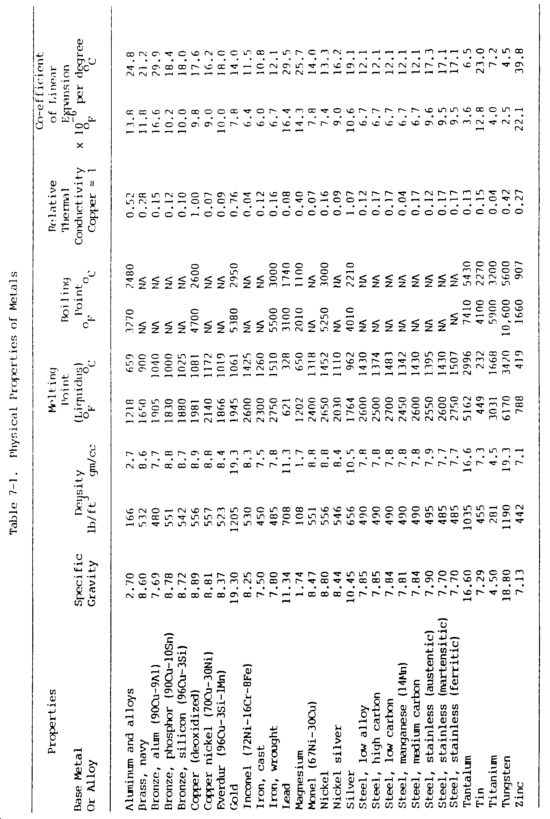
b. Physical Properties. Many of the physical properties of metals determine if and how they can be welded and how they will perform in service. Physical properties of various metals are shown in table 7-1.
(2) Mass or density. Mass or density relates to mass with respect to volume. Commonly known as specific gravity, this property is the ratio of the mass of a given volume of the metal to the mass of the same volume of water at a specified temperature, usually 39°F (4°C). For example, the ratio of weight of one cubic foot of water to one cubic foot of cast iron is the specific gravity of cast iron. This property is measured by grams per cubic millimeter or centimeter in the metric system.
(3) Melting point. The melting point of a metal is important with regard to welding. A metal�s fusibility is related to its melting point, the temperature at which the metal changes from a solid to a molten state. Pure substances have a sharp melting point and pass from a solid state to a liquid without a change in temperature. During this process, however, there is an absorption of heat during melting and a liberation of heat during freezing. The absorption or release of thermal energy when a substance changes state is called its latent heat. Mercury is the only common metal that is in its molten state at normal room temperature. Metals having low melting temperatures can be welded with lower temperature heat sources. The soldering and brazing processes utilize low-temperature metals to join metals having higher melting temperatures.
(4) Boiling point. Boiling point is also an important factor in welding. The boiling point is the temperature at which the metal changes from the liquid state to the vapor state. Some metals, when exposed to the heat of an arc, will vaporize.
(5) Conductivity. Thermal and electrical conductivity relate to the metal�s ability to conduct or transfer heat and electricity. Thermal conductivity, the ability of a metal to transmit heat throughout its mass, is of vital importance in welding, since one metal may transmit heat from the welding area much more quickly than another. The thermal conductivity of a metal indicates the need for preheating and the size of heat source required. Thermal conductivity is usually related to copper. Copper has the highest thermal conductivity of the common metals, exceeded only by silver. Aluminum has approximately half the thermal conductivity of copper, and steels have abut one-tenth the conductivity of copper. Thermal conductivity is measured in calories per square centimeter per second per degree Celsius. Electrical conductivity is the capacity of metal to conduct an electric current. A measure of electrical conductivity is provided by the ability of a metal to conduct the passage of electrical current. Its opposite is resistivity, which is measured in micro-ohms per cubic centimeter at a standardize temperature, usually 20°C. Electrical conductivity is usually considered as a percentage and is related to copper or silver. Temperature bears an important part in this property. As temperature of a metal increases, its conductivity decreases. This property is particularly important to resistance welding and to electrical circuits.
(6) Coefficient of linear thermal expansion. With few exceptions, solids expand when they are heated and contract when they are cooled. The coefficient of linear thermal expansion is a measure of the linear increase per unit length based on the change in temperature of the metal. Expansion is the increase in the dimension of a metal caused by heat. The expansion of a metal in a longitudinal direction is known as the linear expansion. The coefficient of linear expansion is expressed as the linear expansion per unit length for one degree of temperature increase. When metals increase in size, they increase not only in length but also in breadth and thickness. This is called volumetric expansion. The coefficient of linear and volumetric expansion varies over a wide range for different metals. Aluminum has the greatest coefficient of expansion, expanding almost twice as much as steel for the same temperature change. This is important for welding with respect to warpage, wapage control and fixturing, and for welding together dissimilar metals.
(7) Corrosion resistance. Corrosion resistance is the resistance to eating or wearing away by air, moisture, or other agents.
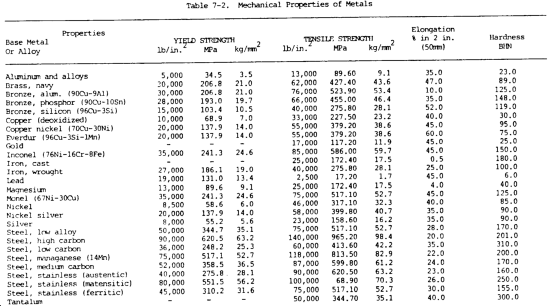
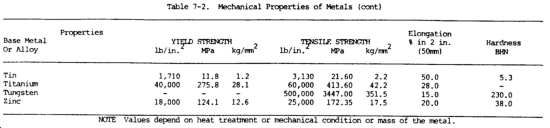
c. Mechanical Properties. The mechanical properties of metals determine the range of usefulness of the metal and establish the service that can be expected. Mechanical properties are also used to help specify and identify the metals. They are important in welding because the weld must provide the same mechanical properties as the base metals being joined. The adequacy of a weld depends on whether or not it provides properties equal to or exceeding those of the metals being joined. The most common mechanical properties considered are strength, hardness, ductility, and impact resistance. Mechanical properties of various metals are shown in table 7-2.
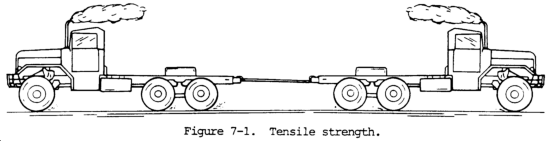
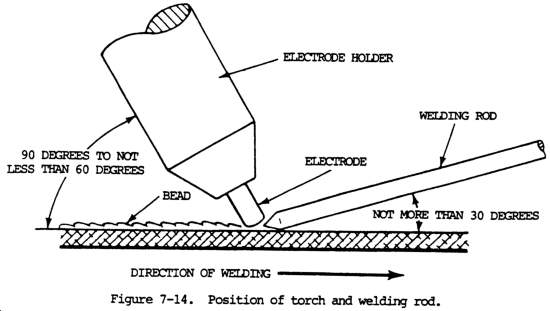
(1) Tensile strength. Tensile strength is defined as the maximum load in tension a material will withstand before fracturing, or the ability of a material to resist being pulled apart by opposing forces. Also known as ultimate strength, it is the maximum strength developed in a metal in a tension test. (The tension test is a method for determining the behavior of a metal under an actual stretch loading. This test provides the elastic limit, elongation, yield point, yield strength, tensile strength, and the reduction in area.) The tensile strength is the value most commonly given for the strength of a material and is given in pounds per square inch (psi) (kiloPascals (kPa)). The tensile strength is the number of pounds of force required to pull apart a bar of material 1.0 in. (25.4 mm) wide and 1.00 in. (25.4 mm) thick (fig. 7-1).
(2) Shear strength. Shear strength is the ability of a material to resist being fractured by opposing forces acting of a straight line but not in the same plane, or the ability of a metal to resist being fractured by opposing forces not acting in a straight line (fig. 7-2).
(3) Fatigue strength. Fatigue strength is the maximum load a material can withstand without failure during a large number of reversals of load. For example, a rotating shaft which supports a weight has tensile forces on the top portion of the shaft and compressive forces on the bottom. As the shaft is rotated, there is a repeated cyclic change in tensile and compressive strength. Fatigue strength values are used in the design of aircraft wings and other structures subject to rapidly fluctuating loads. Fatigue strength is influenced by microstructure, surface condition, corrosive environment, and cold work.
(4) Compressive strength. Compressive strength is the maximum load in compression a material will withstand before a predetermined amount of deformation, or the ability of a material to withstand pressures acting in a given plane (fig. 7-3). The compressive strength of both cast iron and concrete are greater than their tensile strength. For most materials, the reverse is true.
(5) Elasticity. Elasticity is the ability of metal to return to its original size, shape, and dimensions after being deformed, stretched, or pulled out of shape. The elastic limit is the point at which permanent damage starts. The yield point is the point at which definite damage occurs with little or no increase in load. The yield strength is the number of pounds per square inch (kiloPascals) it takes to produce damage or deformation to the yield point.
(6) Modulus of elasticity. The modulus of elasticity is the ratio of the internal stress to the strain produced.
(7) Ductility. The ductility of a metal is that property which allows it to be stretched or otherwise changed in shape without breaking, and to retain the changed shape after the load has been removed. It is the ability of a material, such as copper, to be drawn or stretched permanently without fracture. The ductility of a metal can be determined by the tensile test by determining the percentage of elongation. The lack of ductility is brittleness or the lack of showing any permanent damage before the metal cracks or breaks (such as with cast iron).
(8) Plasticity. Plasticity is the ability of a metal to be deformed extensively without rupture. Plasticity is similar to ductility.
(9) Malleability. Malleability is another form of plasticity, and is the ability of a material to deform permanently under compression without rupture. It is this property which allows the hammering and rolling of metals into thin sheets. Gold, silver, tin, and lead are examples of metals exhibiting high malleability. Gold has exceptional malleability and can be rolled into sheets thin enough to transmit light.
(10) Reduction of area. This is a measure of ductility and is obtained from the tensile test by measuring the original cross-sectional area of a specimen to a cross-sectional area after failure.
(11) Brittleness. Brittleness is the property opposite of plasticity or ductility. A brittle metal is one than cannot be visibly deformed permanently, or one that lacks plasticity.
(12) Toughness. Toughness is a combination of high strength and medium ductility. It is the ability of a material or metal to resist fracture, plus the ability to resist failure after the damage has begun. A tough metal, such as cold chisel, is one that can withstand considerable stress, slowly or suddenly applied, and which will deform before failure. Toughness is the ability of a material to resist the start of permanent distortion plus the ability to resist shock or absorb energy.
(13) Machinability and weldability. The property of machinability and weldability is the ease or difficulty with which a material can be machined or welded.
(14) Abrasion resistance. Abrasion resistance is the resistance to wearing by friction.
(15) Impact resistance. Resistance of a metal to impacts is evaluated in terms of impact strength. A metal may possess satisfactory ductility under static loads, but may fail under dynamic loads or impact. The impact strength of a metal is determined by measuring the energy absorbed in the fracture.
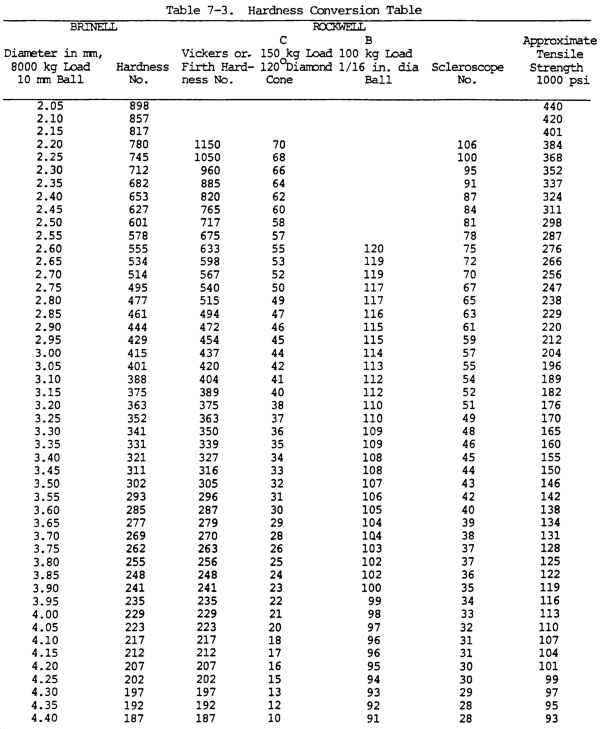
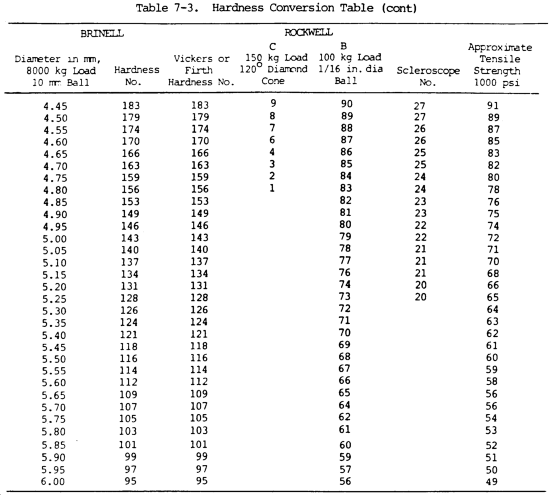
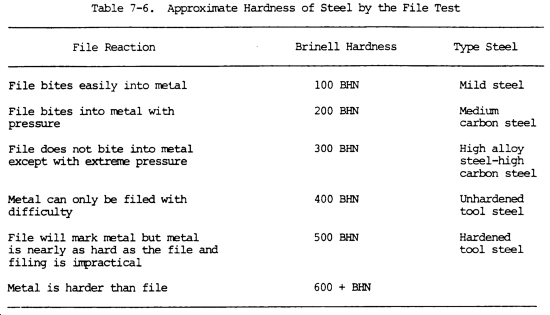
(16) Hardness. Hardness is the ability of a metal to resist penetration and wear by another metal or material. It takes a combination of hardness and toughness to withstand heavy pounding. The hardness of a metal limits the ease with which it can be machined, since toughness decreases as hardness increases. Table 7-3 illustrates hardness of various metals.
(a) Brinell hardness test. In this test, a hardened steel ball is pressed slowly by a known force against the surface of the metal to be tested. The diameter of the dent in the surface is then measured, and the Brinell hardness number (bhn) is determined by from standard tables (table 7-3).
(b) Rockwell hardness test. This test is based upon the difference between the depth to which a test point is driven into a metal by a light load and the depth to which it is driven in by a heavy load. The light load is first applied and then, without moving the piece, the heavy load is applied. The hardness number is automatically indicated on a dial. The letter designations on the Rockwell scale, such as B and C, indicate the type of penetrator used and the amount of heavy load (table 7-3). The same light load is always used.
(c) Scleroscope hardness test. This test measures hardness by letting a diamond-tipped hammer fall by its own weight from a fixed height and rebound from the surface; the rebound is measured on a scale. It is used on smooth surfaces where dents are not desired.
a. General. It is necessary to know the composition of the metal being welded in order to produce a successful weld. Welders and metal workers must be able to identify various metal products so that proper work methods may be applied. For Army equipment, drawings (MWOs) should be available. They must be examined in order to determine the metal to be used and its heat treatment, if required. After some practice, the welder will learn that certain parts of machines or equipment are always cast iron, other parts are usually forgings, and so on.
b. Tests. There are seven tests that can be performed in the shop to identify metals. Six of the different tests are summarized in table 7-4. These should be supplemented by tables 7-1 and 7-2 which present physical and mechanical properties of metal, and table 7-3, which presents hardness data. These tests are as follows:
(1) Appearance test. The appearance test includes such things as color and appearance of machined as well as unmachined surfaces. Form and shape give definite clues as to the identity of the metal. The shape can be descriptive; for example, shape includes such things as cast engine blocks, automobile bumpers, reinforcing rods, I beams or angle irons, pipes, and pipe fittings. Form should be considered and may show how the part was rode, such as a casting with its obvious surface appearance and parting mold lines, or hot rolled wrought material, extruded or cold rolled with a smooth surface. For example, pipe can be cast, in which case it would be cast iron, or wrought, which would normally be steel. Color provides a very strong clue in metal identification. It can distinguish many metals such as copper, brass, aluminum, magnesium, and the precious metals. If metals are oxidized, the oxidation can be scraped off to determine the color of the unoxidized metal. This helps to identify lead, magnesium, and even copper. The oxidation on steel, or rust, is usually a clue that can be used to separate plain carbon steels from the corrosion-resisting steels.
(2) Fracture test. Some metal can be quickly identified by looking at the surface of the broken part or by studying the chips produced with a hammer and chisel. The surface will show the color of the base metal without oxidation. This will be true of copper, lead, and magnesium. In other cases, the coarseness or roughness of the broken surface is an indication of its structure. The ease of breaking the part is also an indication of its ductility of lack of ductility. If the piece bends easily without breaking, it is one of the more ductile metals. If it breaks easily with little or no bending, it is one of the brittle metals.
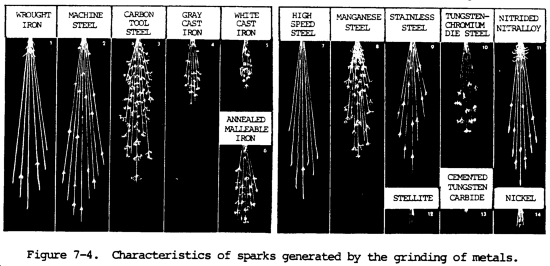
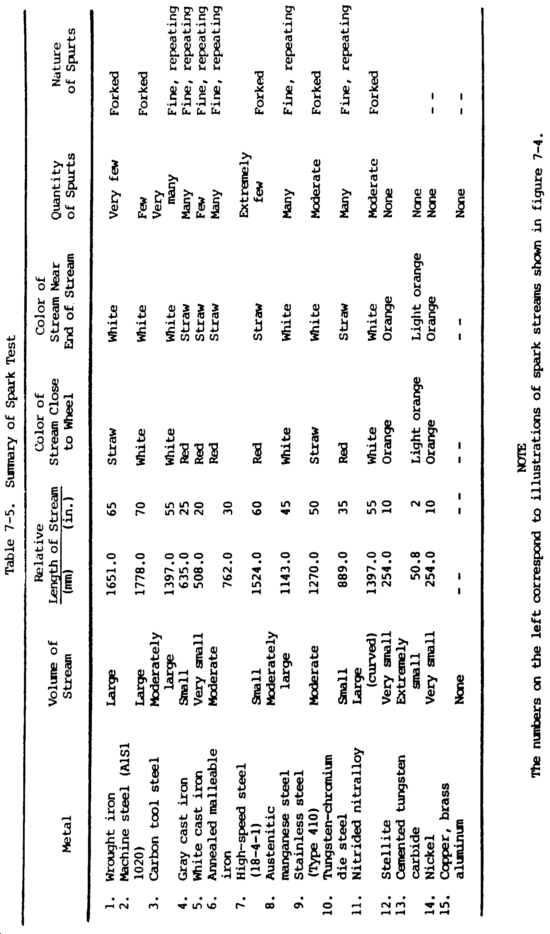
(3) Spark test. The spark test is a method of classifying steels and iron according to their composition by observing the sparks formed when the metal is held against a high speed grinding wheel. This test does not replace chemical analysis, but is a very convenient and fast method of sorting mixed steels whose spark characteristics are known. When held lightly against a grinding wheel, the different kinds of iron and steel produce sparks that vary in length, shape, and color. The grinding wheel should be run to give a surface speed of at least 5000 ft (1525 m) per minute to get a good spark stream. Grinding wheels should be hard enough to wear for a reasonable length of time, yet soft enough to keep a free-cutting edge. Spark testing should be done in subdued light, since the color of the spark is important. In all cases, it is best to use standard samples of metal for the purpose of comparing their sparks with that of the test sample.
(a) Spark testing is not of much use on nonferrous metals such as coppers, aluminums, and nickel-base alloys, since they do not exhibit spark streams of any significance. However, this is one way to separate ferrous and nonferrous metals.
(b) The spark resulting from the test should be directed downward and studied. The color, shape, length, and activity of the sparks relate to characteristics of the material being tested. The spark stream has specific items which can be identified. The straight lines are called carrier lines. They are usually solid and continuous. At the end of the carrier line, they may divide into three short lines, or forks. If the spark stream divides into more lines at the end, it is called a sprig. Sprigs also occur at different places along the carrier line. These are called either star or fan bursts. In some cases, the carrier line will enlarge slightly for a very short length, continue, and perhaps enlarge again for a short length. When these heavier portions occur at the end of the carrier line, they are called spear points or buds. High sulfur creates these thicker spots in carrier lines and the spearheads. Cast irons have extremely short streams, whereas low-carbon steels and most alloy steels have relatively long streams. Steels usually have white to yellow color sparks, while cast irons are reddish to straw yellow. A 0.15 percent carbon steel shows sparks in long streaks with some tendency to burst with a sparkler effect; a carbon tool steel exhibits pronounced bursting; and a steel with 1.00 percent carbon shows brilliant and minute explosions or sparklers. As the carbon content increases, the intensity of bursting increases.
(c) One big advantage of this test is that it can be applied to metal in, all stages, bar stock in racks, machined forgings or finished parts. The spark test is best conducted by holding the steel stationary and touching a high speed portable grinder to the specimen with sufficient pressure to throw a horizontal spark stream about 12.00 in. (30.48 cm) long and at right angles to the line of vision. Wheel pressure against the work is important because increasing pressure will raise the temperature of the spark stream and give the appearance of higher carbon content. The sparks near and around the wheel, the middle of the spark stream, and the reaction of incandescent particles at the end of the spark stream should be observed. Sparks produced by various metals are shown in figure 7-4.
The torch test should be used with discretion, as it may damage the part being tested. Additionally, magnesium may ignite when heated in the open atmosphere.
(4) Torch test. With the oxyacetylene torch, the welder can identify various metals by studying how fast the metal melts and how the puddle of molten metal and slag looks, as well as color changes during heating. When a sharp corner of a white metal part is heated, the rate of melting can be an indication of its identity. If the material is aluminum, it will not melt until sufficient heat has been used because its high conductivity. If the part is zinc, the sharp corner will melt quickly, since zinc is not a good conductor. In the case of copper, if the sharp comer melts, it is normally deoxidized copper. If it does not melt until much heat has been applied, it is electrolytic copper. Copper alloys, if composed of lead, will boil. To distinguish aluminum from magnesium, apply the torch to filings. Magnesium will burn with a sparkling white flame. Steel will show characteristic colors before melting.
(5) Magnetic test. The magnetic test can be quickly performed using a small pocket magnet. With experience, it is possible to judge a strongly magnetic material from a slightly magnetic material. The nonmagnetic materials are easily recognized. Strongly magnetic materials include the carbon and low-alloy steels, iron alloys, pure nickel, and martensitic stainless steels. A slightly magnetic reaction is obtained from Monel and high-nickel alloys and the stainless steel of the 18 chrome 8 nickel type when cold worked, such as in a seamless tube. Nonmagnetic materials include copper-base alloys, aluminum-base alloys, zinc-base alloys, annealed 18 chrome 8 nickel stainless, the magnesium, and the precious metals.
(6) Chisel test. The chip test or chisel test may also be used to identify metals. The only tools required are a banner and a cold chisel. Use the cold chisel to hammer on the edge or corner of the material being examined. The ease of producing a chip is an indication of the hardness of the metal. If the chip is continuous, it is indicative of a ductile metal, whereas if chips break apart, it indicates a brittle material. On such materials as aluminum, mild steel and malleable iron, the chips are continuous. They are easily chipped and the chips do not tend to break apart. The chips for gray cast iron are so brittle that they become small, broken fragments. On high-carbon steel, the chips are hard to obtain because of the hardness of the material, but can be continuous.
(7) Hardness test. Refer to table 7-3 for hardness values of the various metals, and to the above information on the three hardness tests that are commonly used. A less precise hardness test is the file test. A summary of the reaction to filing, the approximate Brinell hardness, and the possible type of steel is shown in table 7-6. A sharp mill file must be used. It is assumed that the part is steel and the file test will help identify the type of steel.
(8) Chemical test. There are numerous chemical tests than can be made in the shop to identify some material. Monel can be distinguished form Inconel by one drop of nitric acid applied to the surface. It will turn blue-green on Monel, but will show no reaction on Inconel. A few drops of a 45 percent phosphoric acid will bubble on low-chromium stainless steels. Magnesium can be distinguished from aluminum using silver nitrate, which will leave a black deposit on magnesium, but not on aluminum. These tests can become complicated, and for this reason are not detailed further here.
c. Color Code for Marking Steel Bars. The Bureau of Standards of the United States Department of Commerce has a color code for making steel bars. The color markings provided in the code may be applied by painting the ends of bars. Solid colors usually mean carbon steel, while twin colors designate alloy and free-cutting steel.
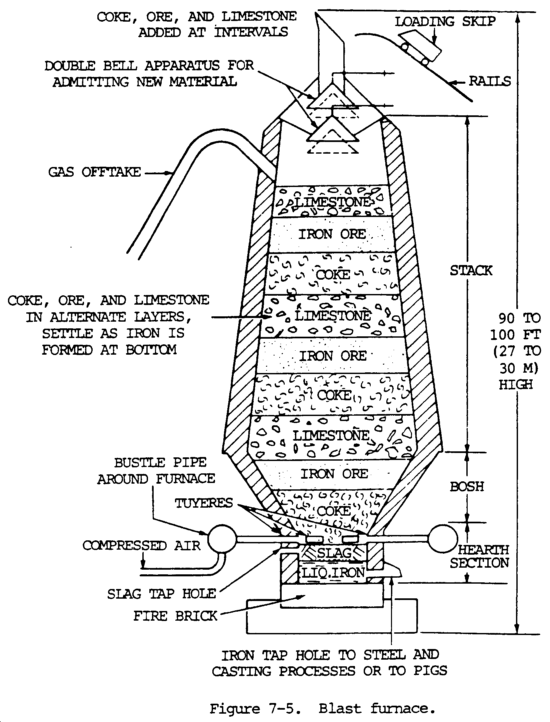
d. Ferrous Metal. The basic substance used to make both steel and cast iron (gray and malleable) is iron. It is used in the form of pig iron. Iron is produced from iron ore that occurs chiefly in nature as an oxide, the two most important oxides being hematite and magnetite. Iron ore is reduced to pig iron in a blast furnace, and the impurities are removed in the form of slag (fig. 7-5). Raw materials charged into the furnace include iron ore, coke, and limestone. The pig iron produced is used to manufacture steel or cast iron.
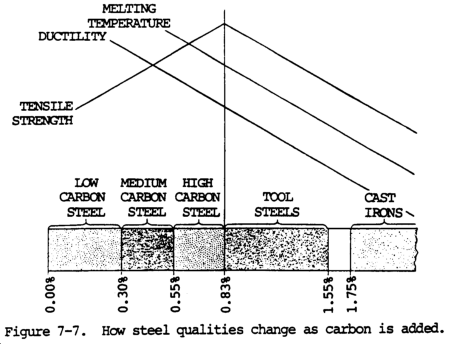
Plain carbon steel consists of iron and carbon. Carbon is the hardening element. Tougher alloy steel contains other elements such as chromium, nickel, and molybdenum. Cast iron is nothing more than basic carbon steel with more carbon added, along with silicon. The carbon content range for steel is 0.03 to 1.7 percent, and 4.5 percent for cast iron.
Steel is produced in a variety of melting furnaces, such as open-hearth, Bessemer converter, crucible, electric-arc, and induction. Most carbon steel is made in open-hearth furnaces, while alloy steel is melted in electric-arc and induction furnaces. Raw materials charged into the furnace include mixtures of iron ore, pig iron, limestone, and scrap. After melting has been completed, the steel is tapped from the furnace into a ladle and then poured into ingots or patterned molds. The ingots are used to make large rectangular bars, which are reduced further by rolling operations. The molds are used for castings of any design.
Cast iron is produced by melting a charge of pig iron, limestone, and coke in a cupola furnace. It is then poured into sand or alloy steel molds. When making gray cast iron castings, the molten metal in the mold is allowed to become solid and cool to room temperature in open air. Malleable cast iron, on the other hand, is made from white cast iron, which is similar in content to gray cast iron except that malleable iron contains less carbon and silicon. White cast iron is annealed for more than 150 hours at temperatures ranging from 1500 to 1700°F (815 to 927°C). The result is a product called malleable cast iron. The desirable properties of cast iron are less than those of carbon steel because of the difference in chemical makeup and structure. The carbon present in hardened steel is in solid solution, while cast iron contains free carbon known as graphite. In gray cast iron, the graphite is in flake form, while in malleable cast iron the graphite is in nodular (rounded) form. This also accounts for the higher mechanical properties of malleable cast iron as compared with gray cast iron.
Iron ore is smelted with coke and limestone in a blast furnace to remove the oxygen (the process of reduction) and earth foreign matter from it. Limestone is used to combined with the earth matter to form a liquid slag. Coke is used to supply the carbon needed for the reduction and carburization of the ore. The iron ore, limestone, and coke are charged into the top of the furnace. Rapid combustion with a blast of preheated air into the smelter causes a chemical reaction, during which the oxygen is removed from the iron. The iron melts, and the molten slag consisting of limestone flux and ash from the coke, together with compounds formed by reaction of the flux with substances present in the ore, floats on the heavier iron liquid. Each material is then drawn off separately (fig. 7-6).
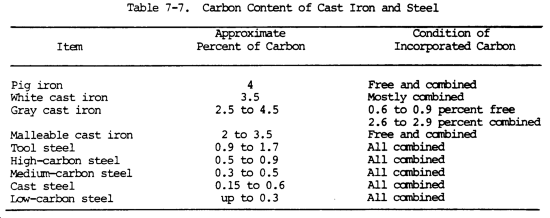
All forms of cast iron, steel, and wrought iron consist of a mixture of iron, carbon, and other elements in small amounts. Whether the metal is cast iron or steel depends entirely upon the amount of carbon in it. Table 7-7 shows this principle.
Cast iron differs from steel mainly because its excess of carbon (more than 1.7 percent) is distributed throughout as flakes of graphite, causing most of the remaining carbon to separate. These particles of graphite form the paths through which failures occur, and are the reason why cast iron is brittle. By carefully controlling the silicon content and the rate of cooling, it is possible to cause any definite amount of the carbon to separate as graphite or to remain combined. Thus, white, gray, and malleable cast iron are all produced from a similar base.
(1) Wrought iron.
(a) General. Wrought iron is almost pure iron. It is made from pig iron in a puddling furnace and has a carbon content of less than 0.08 percent. Carbon and other elements present in pig iron are taken out, leaving almost pure iron. In the process of manufacture, some slag is mixed with iron to form a fibrous structure in which long stringers of slag, running lengthwise, are mixed with long threads of iron. Because of the presence of slag, wrought iron resists corrosion and oxidation, which cause rusting.
(b) Uses. Wrought iron is used for porch railings, fencing, farm implements, nails, barbed wire, chains, modern household furniture, and decorations.
(c) Capabilities. Wrought iron can be gas and arc welded, machined,plated, and is easily formed.
(d) Limitations. Wrought iron has low hardness and low fatigue strength.
(e) Properties. Wrought iron has Brinell hardness number of 105; tensile strength of 35,000 psi; specific gravity of 7.7; melting point of 2750°F (1510°C); and is ductile and corrosion resistant.
(f) Appearance test. The appearance of wrought iron is the same as that of rolled, low-carbon steel.
(g) Fracture test. Wrought iron has a fibrous structure due to threads of slag. As a result, it can be split in the direction in which the fibers run. The metal is soft and easily cut with a chisel, and is quite ductile. When nicked and bent, it acts like rolled steel. However, the break is very jagged due to its fibrous structure. Wrought iron cannot be hardened.
(h) Spark test. When wrought iron is ground, straw-colored sparks form near the grinding wheel, and change to white, forked sparklers near the end of the stream.
(i) Torch test. Wrought iron melts quietly without sparking. It has a peculiar slag coating with white lines that are oily or greasy in appearance.
(2) Cast iron (gray, white, and malleable).
(a) General. Cast iron is a manmade alloy of iron, carbon, and silicon. A portion of the carbon exists as free carbon or graphite. Total carbon content is between 1.7 and 4.5 percent.
(b) Uses. Cast iron is used for water pipes, machine tool castings, transmission housing, engine blocks, pistons, stove castings, etc.
(c) Capabilities. Cast iron may be brazed or bronze welded, gas and arc welded, hardened, or machined.
(d) Limitations. Cast iron must be preheated prior to welding. It cannot be worked cold.
(e) Properties. Cast iron has a Brinell hardness number of 150 to 220 (no alloys) and 300 to 600 (alloyed); tensile strength of 25,000 to 50,000 psi (172,375 to 344,750 kPa) (no alloys) and 50,000 to 100,000 psi (344,750 to 689,500 kPa) (alloyed); specific gravity of 7.6; high compressive strength that is four times its tensile strength; high rigidity; good wear resistance; and fair corrosion resistance.
(f) Gray cast iron. If the molten pig iron is permitted to cool slowly, the chemical compound of iron and carbon breaks up to a certain extent. Much of the carbon separates as tiny flakes of graphite scattered throughout the metal. This graphite-like carbon, as distinguish from combined carbon, causes the gray appearance of the fracture, which characterizes ordinary gray cast iron. Since graphite is an excellent lubricant, and the metal is shot throughout with tiny, flaky cleavages, gray cast iron is easy to machine but cannot withstand a heavy shock. Gray cast iron consists of 90 to 94 percent metallic iron with a mixture of carbon, manganese, phosphorus, sulfur, and silicon. Special high-strength grades of this metal also contain 0.75 to 1.50 percent nickel and 0.25 to 0.50 percent chromium or 0.25 to 1.25 percent molybdenum. Commercial gray iron has 2.50 to 4.50 percent carbon. About 1 percent of the carbon is combined with the iron, while about 2.75 percent remains in the free or graphitic state. In making gray cast iron, the silicon content is usually increased, since this allows the formation of graphitic carbon. The combined carbon (iron carbide), which is a small percentage of the total carbon present in cast iron, is known as cementite. In general, the more free carbon (graphitic carbon) present in cast iron, the lower the combined carbon content and the softer the iron.
1. Appearance test. The unmachined surface of gray cast iron castings is a very dull gray in color and may be somewhat roughened by the sand mold used in casting the part. Cast iron castings are rarely machined all over. Unmachined castings may be ground in places to remove rough edges.
2. Fracture test. Nick a corner all around with a chisel or hacksaw and strike the corner with a sharp blow of the hammer. The dark gray color of the broken surface is caused by fine black specks of carbon present in the form of graphite. Cast iron breaks short when fractured. Small, brittle chips made with a chisel break off as soon as they are formed.
3. Spark test. A small volume of dull-red sparks that follow a straight line close to the wheel are given off when this metal is spark tested. These break up into many fine, repeated spurts that change to a straw color.
4. Torch test. The torch test results in a puddle of molten metal that is quiet and has a jelly like consistency. When the torch flame is raised, the depression in the surface of the molts-puddle disappears instantly. A heavy, tough film forms on the surface as it melts. The molten puddle takes time to harden and gives off no sparks.
(g) White cast iron. When gray cast iron is heated to the molten state, the carbon completely dissolves in the iron, probably combining chemically with it. If this molten metal is cooled quickly, the two elements remain in the combined state, and white cast iron is formed. The carbon in this type of iron measures above 2.5 to 4.5 percent by weight, and is referred to as combined carbon. White cast iron is very hard and brittle, often impossible to machine, and has a silvery white fracture.
(h) Malleable cast iron. Malleable cast iron is made by heating white cast iron from 1400 to 1700°F (760 and 927°C) for abut 150 hours in boxes containing hematite ore or iron scale. This heating causes a part of the combined carbon to change into the free or uncombined state. This free carbon separates in a different way from carbon in gray cast iron and is called temper carbon. It exists in the form of small, rounded particles of carbon which give malleable iron castings the ability to bend before breaking and to withstand shock better than gray cast iron. The castings have properties more like those of pure iron: high strength, ductility, toughness, and ability to resist shock. Malleable cast iron can be welded and brazed. Any welded part should be annealed after welding.
1. Appearance test. The surface of malleable cast iron is very much like gray cast iron, but is generally free from sand. It is dull gray and somewhat lighter in color than gray cast iron.
2. Fracture test. When malleable cast iron is fractured, the central portion of the broken surface is dark gray with a bright, steel-like band at the edges. The appearance of the fracture may best be described as a picture frame. When of good quality, malleable cast iron is much tougher than other cast iron and does not break short when nicked.
3. Spark test. When malleable cast iron is ground, the outer, bright layer gives off bright sparks like steel. As the interior is reached, the sparks quickly change to a dull-red color near the wheel. These sparks from the interior section are very much like those of cast iron; however, they are somewhat longer and are present in large volume.
4. Torch test. Molten malleable cast iron boils under the torch flame. After the flame has been withdrawn, the surface will be full of blowholes. When fractured, the melted parts are very hard and brittle, having the appearance of white cast iron (they have been changed to white or chilled iron by melting and fairly rapid cooling). The outside, bright, steel-like band gives off sparks, but the center does not.
(3) Steel.
(a) General. A form of iron, steel contains less carbon than cast iron, but considerably more than wrought iron. The carbon content is from 0.03 to 1.7 percent. Basic carbon steels are alloyed with other elements, such as chromium and nickel, to increase certain physical properties of the metal.
(b) Uses. Steel is used to make nails, rivets, gears, structural steel, roles, desks, hoods, fenders, chisels, hammers, etc.
(c) Capabilities. Steel can be machined, welded, and forged, all to varying degrees, depending on the type of steel.
(d) Limitations. Highly alloyed steel is difficult to produce.
(e) Properties. Steel has tensile strength of 45,000 psi (310,275 kPa) for low-carbon steel, 80,000 psi (551,600 kPa) for medium-carbon steel, 99,000 psi (692,605 kPa) for high-carbon steel, and 150,000 psi (1,034,250 kPa) for alloyed steel; and a melting point of 2800° F (1538°C).
(f) Low-carbon steel (carbon content up to 0.30 percent. This steel is soft and ductile, and can be rolled, punched, sheared, and worked when either hot or cold. It is easily machined and can readily be welded by all methods. It does not harden to any great amount; however, it can easily be case hardened.
1. Appearance test. The appearance of the steel depends upon the method of preparation rather than upon composition. Cast steel has a relatively rough, dark-gray surface, except where it has been machined. Rolled steel has fine surface lines running in one direction. Forged steel is usually recognizable by its shape, hammer marks, or fins.
2. Fracture test. When low-carbon steel is fractured, the color is bright crystalline gray. It is tough to chip or nick. Low carbon steel, wrought iron, and steel castings cannot be hardened.
3. Spark test. The steel gives off sparks in long yellow-orange streaks, brighter than cast iron, that show some tendency to burst into white, forked sparklers.
4. Torch test. The steel gives off sparks when melted, and hardens almost instantly.
(g) Medium-carbon steel (carbon content ranging from 0.30 to 0.50 percent). This steel may be heat-treated after fabrication. It is used for general machining and forging of parts that require surface hardness and strength. It is made in bar form in the cold-rolled or the normalized and annealed condition. During welding, the weld zone will become hardened if cooled rapidly and must be stress-relieved after welding.
(h) High-carbon steel (carbon content ranging from 0.50 to 0.90 percent). This steel is used for the manufacture of drills, taps, dies, springs, and other machine tools and hand tools that are heat treated after fabrication to develop the hard structure necessary to withstand high shear stress and wear. It is manufactured in bar, sheet, and wire forms, and in the annealed or normalized condition in order to be suitable for machining before heat treatment. This steel is difficult to weld because of the hardening effect of heat at the welded joint.
1. Appearance test. The unfinished surface of high-carbon steel is dark gray and similar to other steel. It is more expensive, and is usually worked to produce a smooth surface finish.
2. Fracture test. High-carbon steel usually produces a very fine-grained fracture, whiter than low-carbon steel. Tool steel is harder and more brittle than plate steel or other low-carbon material. High-carbon steel can be hardened by heating to a good red and quenching in water.
3. Spark test. High-carbon steel gives off a large volume of bright yellow-orange sparks.
4. Torch test. Molten high-carbon steel is brighter than lowcarbon steel, and the melting surface has a porous appearance. It sparks more freely than low-carbon (mild) steels, and the sparks are whiter.
(i) High carbon tool steel. Tool steel (carbon content ranging from 0.90 to 1.55 percent) is used in the manufacture of chisels, shear blades, cutters, large taps, wood-turning tools, blacksmith�s tools, razors, and similar parts where high hardness is required to maintain a sharp cutting edge. It is difficult to weld due to the high carbon content. A spark test shows a moderately large volume of white sparks having many fine, repeating bursts.
(a) General. Welding is difficult on steel castings containing over 0.30 percent carbon and 0.20 percent silicon. Alloy steel castings containing nickel, molybdenum, or both of these metals, are easily welded if the carbon content is low. Those containing chromium or vanadium are more difficult to weld. Since manganese steel is nearly always used in the form of castings, it is also considered with cast steel. Its high resistance to wear is its most valuable property.
(b) Appearance test. The surface of cast steel is brighter than cast or malleable iron and sometimes contains small, bubble-like depressions.
(c) Fracture test. The color of a fracture in cast steel is bright crystalline gray. This steel is tough and does not break short. Steel castings are tougher than malleable iron, and chips made with a chisel curl up more. Manganese steel, however, is so tough that is cannot be cut with a chisel nor can it be machined.
(d) Spark test. The sparks created from cast steel are much brighter than those from cast iron. Manganese steel gives off marks that explode, throwing off brilliant sparklers at right angles to the original-path of the spark:
(e) Torch test. When melted, cast steel sparks and hardens quickly.
(5) Steel forgings.
(a) General. Steel forgings may be of carbon or alloy steels. Alloy steel forgings are harder and more brittle than low carbon steels.
(b) Appearance test. The surface of steel forgings is smooth. Where the surface of drop forgings has not been finished, there will be evidence of the fin that results from the metal squeezing out between the two forging dies. This fin is removed by the trimming dies, but enough of the sheared surface remains for identification. All forgings are covered with reddish brown or black scale, unless they have been purposely cleaned.
(c) Fracture test. The color of a fracture in a steel forging varies from bright crystalline to silky gray. Chips are tough; and when a sample is nicked, it is harder to break than cast steel and has a finer grain. Forgings may be of low-or high-carbon steel or of alloy steel. Tool steel is harder and more brittle than plate steel or other low-carbon material. The fracture is usually whiter and finer grained. Tool steel can be hardened by heating to a good red and then quenching in water. Low-carbon steel, wrought iron, and steel castings cannot be usefully hardened.
(d) Spark test. The sparks given off are long, yellow-orange streamers and are typical steel sparks. Sparks from high-carbon steel (machinery and tool steel) are much brighter than those from low-carbon steel.
(e) Torch test. Steel forgings spark when melted, and the sparks increase in number and brightness as the carbon content becomes greater.
(6) Alloy steel.
(a) General. Alloy steel is frequently recognizable by its use. There are many varieties of alloy steel used in the manufacture of Army equipment. They have greater strength and durability than carbon steel, and a given strength is secured with less material weight. Manganese steel is a special alloy steel that is always used in the cast condition (see cast steel, above).
Nickel, chromium, vanadium, tungsten, molybdenum, and silicon are the most common elements used in alloy steel.
1. Chromium is used as an alloying element in carbon steels to increase hardenability, corrosion resistance, and shock resistance. It imparts high strength with little loss in ductility.
2. Nickel increases the toughness, strength, and ductility of steels, and lowers the hardening temperatures so than an oil quench, rather than a water quench, is used for hardening.
3. Manganese is used in steel to produce greater toughness, wear resistance, easier hot rolling, and forging. An increase in manganese content decreases the weldability of steel.
4. Molybdenum increases hardenability, which is the depth of hardening possible through heat treatment. The impact fatigue property of the steel is improved with up to 0.60 percent molybdenum. Above 0.60 percent molybdenum, the impact fatigue property is impaired. Wear resistance is improved with molybdenum content above 0.75 percent. Molybdenum is sometimes combined with chromium, tungsten, or vanadium to obtain desired properties.
5. Titanium and columbium (niobium) are used as additional alloying agents in low-carbon content, corrosion resistant steels. They support resistance to intergranular corrosion after the metal is subjected to high temperatures for a prolonged time period.
6. Tungsten, as an alloying element in tool steel, produces a fine, dense grain when used in small quantities. When used in larger quantities, from 17 to 20 percent, and in combination with other alloys, it produces a steel that retains its hardness at high temperatures.
7. Vanadium is used to help control grain size. It tends to increase hardenability and causes marked secondary hardness, yet resists tempering. It is also added to steel during manufacture to remove oxygen.
8. Silicon is added to steel to obtain greater hardenability and corrosion resistance, and is often used with manganese to obtain a strong, tough steel. High speed tool steels are usually special alloy compositions designed for cutting tools. The carbon content ranges from 0.70 to 0.80 percent. They are difficult to weld except by the furnace induction method.
9. High yield strength, low alloy structural steels (often referred to as constructional alloy steels) are special low carbon steels containing specific small amounts of alloying elements. These steels are quenched and tempered to obtain a yield strength of 90,000 to 100,000 psi (620,550 to 689,500 kPa) and a tensile strength of 100,000 to 140,000 psi (689,500 to 965,300 kPa), depending upon size and shape. Structural members fabricated of these high strength steels may have smaller cross sectional areas than common structural steels, and still have equal strength. In addition, these steels are more corrosion and abrasion resistant. In a spark test, this alloy appears very similar to the low carbon steels.
NOTE
This type of steel is much tougher than low carbon steels, and shearing machines must have twice the capacity required for low carbon steels.
(b) Appearance test. Alloy steel appear the same as drop-forged steel.
(c) Fracture test. Alloy steel is usually very close grained; at times the fracture appears velvety.
(d) Spark test. Alloy steel produces characteristic sparks both in color and shape. Some of the more common alloys used in steel and their effects on the spark stream are as follows:
1. Chromium. Steels containing 1 to 2 percent chromium have no outstanding features in the spark test. Chromium in large amounts shortens the spark stream length to one-half that of the same steel without chromium, but does not appreciably affect the stream�s brightness. Other elements shorten the stream to the same extent and also make it duller. An 18 percent chromium, 8 percent nickel stainless steel produces a spark similar to that of wrought iron, but only half as long. Steel containing 14 percent chromium and no nickel produces a shorter version of the low-carbon spark. An 18 percent chromium, 2 percent carbon steel (chromium die steel) produces a spark similar to that of carbon tool steel, but one-third as long.
2. Nickel. The nickel spark has a short, sharply defined dash of brilliant light just before the fork. In the amounts found in S. A. E. steels, nickel can be recognized only when the carbon content is so low that the bursts are not too noticeable.
3. High chromium-nickel alloy (stainless) steels. The sparks given off during a spark test are straw colored near the grinding wheel and white near the end of the streak. There is a medium volume of streaks having a moderate number of forked bursts.
4. Manganese. Steel containing this element produces a spark similar to a carbon steel spark. A moderate increase in manganese increases the volume of the spark stream and the force of the bursts. Steel containing more than the normal amount of manganese will spark in a manner similar to high-carbon steel with low manganese content.
5. Molybdenum. Steel containing this element produces a characteristic spark with a detached arrowhead similar to that of wrought iron. It can be seen even in fairly strong carbon bursts. Molybdenum alloy steel contains nickel, chromium, or both.
6. Molybdenum with other elements. When molybdenum and other elements are substituted for some of the tungsten in high-speed steel, the spark stream turns orange. Although other elements give off a red spark, there is enough difference in their color to tell them from a tungsten spark.
7. Tungsten. Tungsten will impart a dull red color to the spark stream near the wheel. It also shortens the spark stream, decreases the size, or completely eliminates the carbon burst. Steel containing 10 percent tungsten causes short, curved, orange spear points at the end of the carrier lines. Still lower tungsten content causes small white bursts to appear at the end of the spear point. Carrier lines may be anything from dull red to orange in color, depending on the other elements present, if the tungsten content is not too high.
8. Vanadium. Alloy steels containing vanadium produce sparks with a detached arrowhead at the end of the carrier line similar to those arising from molybdenum steels. The spark test is not positive for vanadium steels.
9. High speed tool steels. A spark test in these steels will impart a few long; forked sparks which are red near the wheel, and straw-colored near the end of the spark stream.
(7) Special steel. Plate steel is used in the manufacture of built-up welded structures such as gun carriages. In using nickel plate steel, it has been found that commercial grades of low-alloy structural steel of not over 0.25 percent carbon, and several containing no nickel at all, are better suited to welding than those with a maximum carbon content of 0.30 percent. Armorplate, a low carbon alloyed steel, is an example of this kind of plate. Such plate is normally used in the "as rolled" condition. Electric arc welding with a covered electrode may require preheating of the metal, followed by a proper stress-relieving heat treatment (post heating), to produce a structure in which the welded joint has properties equal to those of the plate metal.
e. Nonferrous metal.
(1) Aluminum (Al).
(a) General. Aluminum is a lightweight, soft, low strength metal which can easily be cast, forged, machined, formed, and welded. It is suitable only in low temperature applications, except when alloyed with specific elements. Commercial aluminum alloys are classified into two groups, wrought alloys and cast alloys. The wrought alloy group includes those alloys which are designed for mill products whose final physical forms are obtained by working the metal mechanically. The casting alloy group includes those alloys whose final shapes are obtained by allowing the molten metal to solidify in a mold.
(b) Uses. Aluminum is used as a deoxidizer and alloying agent in the manufacture of steel. Castings, pistons, torque converter pump housings, aircraft structures, kitchen utensils, railways cars, and transmission lines are made of aluminum.
(c) Capabilities. Aluminum can be cast, forged, machined, formed, and welded.
(d) Limitations. Direct metal contact of aluminum with copper and copper alloys should be avoided. Aluminum should be used in low-temperature applications.
(e) Properties. Pure aluminum has a Brinell hardness number of 17 to 27; tensile strength of 6000 to 16,000 psi (41,370 to 110,320 kPa); specific gravity of 2.7; and a melting point of 1220°F (660°C). Aluminum alloys have a Brinell hardness number of 100 to 130, and tensile strength of 30,000 to 75,000 psi (206,850 to 517,125 kPa). Generally, aluminum and aluminum alloys have excellent heat conductivity; high electrical conductivity (60 percent that of copper, volume for volume; high strength/weight ratio at room temperature; and unfairly corrosion resistant.
(f) Appearance test. Aluminum is light gray to silver in color, very bright when polished, dull when oxidized, and light in weight. Rolled and sheet aluminum materials are usually pure metal. Castings are alloys of aluminum with other metals, usually zinc, copper, silicon, and sometimes iron and magnesium. Wrought aluminum alloys may contain chromium, silicon, magnesium, or manganese. Aluminum strongly resembles magnesium in appearance. Aluminum is distinguished from magnesium by the application of a drop of silver nitrate solution on each surface. The silver nitrate will not react with the aluminum, but leaves a black deposit of silver on the magnesium.
(g) Fracture test. A fracture in rolled aluminum sections shows a smooth, bright structure. A fracture in an aluminum casting shins a bright crystalline structure.
(h) Spark test. No sparks are given off from aluminum.
(i) Torch test. Aluminum does not turn red before melting. It holds its shape until almost molten, then collapses (hot shorts) suddenly. A heavy film of white oxide forms instantly on the molten surface.
(2) Chromium (Cr).
(a) General. Chromium is an alloying agent used in steel, cast iron, and nonferrous alloys of nickel, copper, aluminum, and cobalt. It is hard, brittle, corrosion resistant, can be welded, machined, forged, and is widely used in electroplating. Chromium is not resistant to hydrochloric acid and cannot be used in its pure state because of its difficulty to work.
(b) Uses. Chromium is one of the most widely used alloys. It is used as an alloying agent in steel and cast iron (0.25 to 0.35 percent) and in nonferrous alloys of nickel, copper, aluminum, and cobalt. It is also used in electroplating for appearance and wear, in powder metallurgy, and to make mirrors and stainless steel.
(c) Capabilities. Chromium alloys can be welded, machined, and forged. Chromium is never used in its pure state.
(d) Limitations. Chromium is not resistant to hydrochloric acid, and cannot be used in the pure state because of its brittleness and difficulty to work.
(e) Properties (pure). Chromium has a specific gravity of 7.19; a melting point of 3300°F (1816°C); Brinell hardness number of 110 to 170; is resistant to acids other than hydrochloric; and is wear, heat, and corrosion resistant.
(3) Cobalt (Co).
(a) General. Cobalt is a hard, white metal similar to nickel in appearance, but has a slightly bluish cast.
(b) Uses. Cobalt is mainly used as an alloying element in permanent and soft magnetic materials, high-speed tool bits and cutters, high-temperature, creep-resisting alloys, and cemented carbide tools, bits, and cutters. It is also used in making insoluble paint pigmnts and blue ceramic glazes. In the metallic form, cobalt does not have many uses. However, when combined with other elements, it is used for hard facing materials.
(c) Capabilities. Cobalt can be welded, machined (limited), and cold-drawn.
(d) Limitations. Cobalt must be machined with cemented carbide cutters. Welding high carbon cobalt steel often causes cracking.
(e) Properties. Pure cobalt has a tensile strength of 34,000 psi (234,430 kPa); Brinell hardness number of 125; specific gravity of 8.9; and a melting point of 2720°F (1493°C). Cobalt alloy (Stellite 21) has a tensile strength of 101,000 psi (696,395 kPa) and is heat and corrosion resistant.
(4) Copper (Cu).
(a) General. Copper is a reddish metal, is very ductile and malleable, and has high electrical and heat conductivity. It is used as a major element in hundreds of alloys. Commercially pure copper is not suitable for welding. Though it is very soft, it is very difficult to machine due to its high ductility. Beryllium copper contains from 1.50 to 2.75 percent beryllium. It is ductile when soft, but loses ductility and gains tensile strength when hardened. Nickel copper contains either 10, 20, or 30 percent nickel. Nickel alloys have moderately high to high tensile strength, which increases with the nickel content. They are moderately hard, quite tough, and ductile. They are very resistant to the erosive and corrosive effects of high velocity sea water, stress corrosion, and corrosion fatigue. Nickel is added to copper zinc alloys (brasses) to lighten their color; the resultant alloys are called nickel silver. These alloys are of two general types, one type containing 65 percent or more copper and nickel combined, the other containing 55 to 60 percent copper and nickel combined. The first type can be cold worked by such operations as deep drawing, stamping, and spinning. The second type is much harder end is not processed by any of the cold working methods. Gas welding is the preferred process for joining copper and copper alloys.
(b) Uses. The principal use of commercially pure copper is in the electrical industry where it is made into wire or other such conductors. It is also used in the manufacture of nonferrous alloys such as brass, bronze, and Monel metal. Typical copper products are sheet roofing, cartridge cases, bushings, wire, bearings, and statues.
(c) Capababilities. Copper can be forged, cast, and cold worked. It can also be welded, but its machinability is only fair. Copper alloys can be welded.
(d) Limitations. Electrolytic tough pitch copper cannot be welded satisfactorily. Pure copper is not suitable for welding and is difficult to machine due to its ductility.
(e) Properties. Pure copper is nonmagnetic; has a Brinell hardness number of 60 to 110; a tensile strength of 32,000 to 60,000 psi (220,640 to 413,700 kPa); specific gravity of 8.9; melting point of 1980°F (1082°C); and is corrosion resistant. Copper alloys have a tensile strength of 50,000 to 90,000 psi (344,750 to 620,550 kPa) and a Brinell hardness number of 100 to 185.
(f) Appearance test. Copper is red in color when polished, and oxidizes to various shades of green.
(g) Fracture test. Copper presents a smooth surface when fractured, which is free from crystalline appearance.
(h) Spark test. Copper gives off no sparks.
(i) Torch test. Because copper conducts heat rapidly, a larger flame is required to produce fusion of copper than is needed for the same size piece of steel. Copper melts suddenly and solidifies instantly. Copper alloy, containing small amounts of other metals, melts more easily and solidifies more slowly than pure copper.
(j) Brass and bronze. Brass, an alloy of copper and zinc (60 to 68 percent copper and 32 to 40 percent zinc), has a low melting point and high heat conductivity. There are several types of brass, such as naval, red, admiralty, yellow, and commercial. All differ in copper and zinc content; may be alloyed with other elements such as lead, tin, manganese, or iron; have good machinability; and can be welded. Bronze is an alloy of copper and tin and may contain lead, zinc, nickel, manganese, or phosphorus. It has high strength, is rust or corrosion resistant, has good machinability, and can be welded.
1. Appearance test. The color of polished brass and bronze varies with the composition from red, almost like copper, to yellow brass. They oxidize to various shades of green, brown, or yellow.
2. Fracture test. The surface of fractured brass or bronze ranges from smooth to crystalline, depending upon composition and method of preparation; i. e., cast, rolled, or forged.
3. Spark test. Brass and bronze give off no sparks.
4. Torch test. Brass contains zinc, which gives off white fumes when it is melted. Bronze contains tin. Even a slight amount of tin makes the alloy flow very freely, like water. Due to the small amount of zinc or tin that is usually present, bronze may fume slightly, but never as much as brass.
(k) Aluminum bronze.
1. Appearance test. When polished, aluminum bronze appears a darker yellow than brass.
2. Fracture test. Aluminum bronze presents a smooth surface when fractured.
3. Spark test. Aluminum bronze gives off no sparks.
4. Torch test. Welding aluminum bronze is very difficult. The surface is quickly covered with a heavy scum that tends to mix with the metal and is difficult to remove.
(5) Lead (Pb).
CAUTION
Lead dust and fumes are poisonous. Exercise extreme care when welding lead, and use personal protective equipment as described in chapter 2.
(a) General. Lead is a heavy, soft, malleable metal with low melting point, low tensile strength, and low creep strength. It is resistant to corrosion from ordering atmosphere, moisture, and water, and is effective against many acids. Lead is well suited for cold working and casting. The low melting point of lead makes the correct welding rod selection very important.
(b) Uses. Lead is used mainly in the manufacture of electrical equipment such as lead-coated power and telephone cables, and storage batteries. It is also used in building construction in both pipe and sheet form, and in solder. Zinc alloys are used in the manufacture of lead weights, bearings, gaskets, seals, bullets, and shot. Many types of chemical compounds are produced from lead; among these are lead carbonate (paint pigment) and tetraethyl lead (antiknock gasoline). Lead is also used for X-ray protection (radiation shields). Lead has more fields of application than any other metal.
(c) Capabilities. Lead can be cast, cold worked, welded, and machined. It is corrosion, atmosphere, moisture, and water resistant, and is resistant to many acids.
(d) Limitations. Lead has low strength with heavy weight. Lead dust and fumes are very poisonous.
(e) Properties. Pure lead has tensile strength of 2500 to 3000 psi (17,237.5 to 20,685 kPa); specific gravity of 11.3; and a melting point of 620°F (327°C). Alloy lead B32-467 has tensile strength of 5800 psi (39,991 kPa). Generally, lead has low electrical conductivity; is self-lubricating; is malleable; and is corrosion resistant.
(6) Magnesium (Mg).
(a) General. Magnesium is an extremely light metal, is white in color, has a low melting point, excellent machinability, and is weldable. Welding by either the arc or gas process requires the use of a gaseous shield. Magnesium is moderately resistant to atmospheric exposure, many chemicals such as alkalies, chromic and hydrofluoric acids, hydrocarbons, and most alcohols, phenols, esters, and oils. It is nonmagnetic. Galvanic corrosion is an important factor in any assembly with magnesium.
(b) Uses. Magnesium is used as a deoxidizer for brass, bronze, nickel, and silver. Because of its light weight, it is used in many weight-saving applications, particularly in the aircraft industry. It is also used in the manufacture and use of fireworks for railroad flares and signals, and for military purposes. Magnesium castings are used for engine housings, blowers, hose pieces, landing wheels, and certain parts of the fuselage of aircraft. Magnesium alloy materials are used in sewing machines, typewriters, and textile machines.
(c) Capabilities. Magnesium can be forged, cast, welded, and machined.
(d) Limitations. Magnesium in fine chip form will ignite at low temperatures (800 to 1200°F (427 to 649°C)). The flame can be mothered with suitable materials such as carbon dioxide (CO2), foam, and sand.
(e) Properties. Pure magnesium has tensile strength of 12,000 psi (82,740 kPa) (cast) and tensile strength of 37,000 psi (255,115 kPa) (rolled); Brinell hardness number of 30 (cast) and 50 (rolled); specific gravity of 1.7; and a melting point of 1202°F (650°C). Magnesium alloy has Brinell hardness number of 72 (hard) and 50 (forged); and tensile strength of 42,000 psi (289,590 kPa) (hard) and 32,000 psi (220,640 kPa) (forged).
(f) Appearance test. Magnesium resembles aluminum in appearance. The polished surface is silver-white, but quickly oxidizes to a grayish film. Like aluminum, it is highly corrosion resistant and has a good strength-to-weight ratio, but is lighter in weight than aluminum. It has a very low kindling point and is not very weldable, except when it is alloyed with manganese and aluminum. Magnesium is distinguished from aluminum by the use of a silver nitrate solution. The solution does not react with aluminum, but leaves a black deposit of silver on magnesium. Magnesium is produced in large quantities from sea water. It has excellent machinability, but special care must be used when machining because of its low kindling point.
(g) Fracture test. Magnesium has a rough surface with a fine grain structure.
(h) Spark test. No sparks are given off.
CAUTION
Magnesium may ignite and burn when heated in the open atmosphere.
(i) Torch test. Magnesium oxidizes rapidly when heated in open air, producing an oxide film which is insoluble in the liquid metal. A fire may result when magnesium is heated in the open atmosphere. As a safety precaution, magnesium should be melted in an atmosphere of inert gas.
(7) Manganese (Mn).
(a) General. Pure manganese has a relatively high tensile strength, but is very brittle. Manganese is used as an alloying agent in steel to deoxidize and desulfurize the metal. In metals other than steel, percentages of 1 to 15 percent manganese will increase the toughness and the hardenability of the metal involved.
(b) Uses. Manganese is used mainly as an alloying agent in making steel to increase tensile strength. It is also added during the steel-making process to remove sulfur as a slag. Austenitic manganese steels are used for railroad track work, power shovel buckets, and rock crushers. Medium-carbon manganese steels are used to make car axles and gears.
(c) Capabilities. Manganese can be welded, machined, and cold-worked.
(d) Limitations. Austenitic manganese steels are best machined with cemented carbide, cobalt, and high-speed steel cutters.
(e) Properties. Pure manganese has tensile strength of 72,000 psi (496,440 kPa) (quenched) Brinell hardness number of 330; specific gravity of 7.43: a melting point of 2270°F (1243°C); and is brittle. Manganese alloy has a tensile strength of 110,000 psi (758,450 kPa). Generally, manganese is highly polishable and brittle.
(8) Molybdenum (Mo).
(a) General. Pure molybdenum has a high tensile strength and is very resistant to heat. It is principally used as an alloying agent in steel to increase strength, hardenability, and resistance to heat.
(b) Uses. Molybdenum is used mainly as an alloy. Heating elements, switches, contacts, thermocouplers, welding electrodes, and cathode ray tubes are made of molybdenum.
(c) Capabilities. Molybdenum can be swaged, rolled, drawn, or machined.
(d) Limitations. Molybdenum can only be welded by atomic hydrogen arc, or butt welded by resistance heating in vacuum. It is attacked by nitric acid, hot sulfuric acid, and hot hydrochloric acid.
(e) Properties. Pure molybdenum has a tensile strength of 100,000 psi (689,500 kPa) (sheet) and 30,000 Psi (206,850 kPa) (wire); Brinell hardness number of 160 to 185; specific gravity of 10.2; meting point of 4800°F (2649°C); retains hardness and strength at high temperatures; and is corrosion resistant.
(9) Nickel (Ni).
(a) General. Nickel is a hard, malleable, ductile metal. As an alloy, it will increase ductility, has no effect on grain size, lowers the critical point for heat treatment, aids fatigue strength, and increases impact values in low temperature operations. Both nickel and nickel alloys are machinable and are readily welded by gas and arc methods.
(b) Uses. Nickel is used in making alloys of both ferrous and nonferrous metal. Chemical and food processing equipment, electrical resistance heating elements, ornamental trim, and parts that must withstand elevated temperatures are all produced from nickel-containing metal. Alloyed with chromium, it is used in the making of stainless steel.
(c) Capabilities. Nickel alloys are readily welded by either the gas or arc methods. Nickel alloys can be machined, forged, cast, and easily formed.
(d) Limitations. Nickel oxidizes very slowly in the presence of moisture or corrosive gases.
(e) Properties. Pure nickel has tensile strength of 46,000 psi (317,170 kPa); Brinell hardness number 220; specific gravity of 8.9; and melting point of 2650°F (1454°C). Nickel alloys have Brinell hardness number of 140 to 230. Monel-forged nickel has tensile strength of 100,000 psi (689,500 kPa), and high strength and toughness at high temperatures.
(f) Appearance. Pure nickel has a grayish white color.
(g) Fracture. The fracture surface of nickel is smooth and fine grained.
(h) Spark test. In a spark test, nickel produces a very small amount of short, orange streaks which are generally wavy.
(i) Monel metal. Monel metal is a nickel alloy of silver-white color containing about 67.00 percent nickel, 29.00 to 80.00 percent copper, 1.40 percent iron, 1.00 percent manganese, 0.10 percent silicon, and 0.15 percent carbon. In appearance, it resembles untarnished nickel. After use, or after contact with chemical solutions, the silver-white color takes on a yellow tinge, and some of the luster is lost. It has a very high resistance to corrosion and can be welded.
(10) Tin (Sn).
(a) General. Tin is a very soft, malleable, somewhat ductile, corrosion resistant metal having low tensile strength and high crystalline structure. It is used in coating metals to prevent corrosion.
(b) Uses. The major application of tin is in coating steel. It serves as the best container for preserving perishable focal. Tin, in the form of foil, is often used in wrapping food products. A second major use of tin is as an alloying element. Tin is alloyed with copper to produce tin brass and bronze, with lead to produce solder, and with antimony and lead to form babbitt.
(c) Capabilities. Tin can be die cast, cold worked (extruded), machined, and soldered.
(d) Limitations. Tin is not weldable.
(e) Properties. Pure tin has tensile strength of 2800 psi (19,306 kPa); specific gravity of 7.29; melting point of 450°F (232°C); and is corrosion resistant. Babbitt alloy tin has tensile strength of 10,000 psi (68,950 kPa) and Brinell hardness number of 30.
(f) Appearance. Tin is silvery white in color.
(g) Fracture test. The fracture surface of tin is silvery white and fairly smooth.
(h) Spark test. Tin gives off no sparks in a spark test.
(i) Torch test. Tin melts at 450°F (232°C), and will boil under the torch.
(11) Titanium (Ti).
(a) General. Titanium is a very soft, silvery white, medium-strength metal having very good corrosion resistance. It has a high strength to weight ratio, and its tensile strength increases as the temperature decreases. Titanium has low impact and creep strengths, as well as seizing tendencies, at temperatures above 800°F (427°C).
(b) Uses. Titanium is a metal of the tin group which occurs naturally as titanium oxide or in other oxide forms. The free element is separated by heating the oxide with aluminum or by the electrolysis of the solution in calcium chloride. Its most important compound is titanium dioxide, which is used widely in welding electrode coatings. It is used as a stabilizer in stainless steel so that carbon will not be separated during the welding operation. It is also used as an additive in alloying aluminum, copper, magnesium, steel, and nickel; making powder for fireworks; and in the manufacture of turbine blades, aircraft firewalls, engine nacelles, frame assemblies, ammunition tracks, and mortar base plates.
(c) Capabilities. Titanium can be machined at low speeds and fast feeds; formal; spot-and seam-welded, and fusion welded using inert gas.
(d) Limitations. Titanium has low impact strength, and low creep strength at high temperatures (above 800°F (427°C)). It can only be cast into simple shapes, and it cannot be welded by any gas welding process because of its high attraction for oxygen. Oxidation causes this metal to become quite brittle. The inert gas welding process is recommended to reduce contamination of the weld metal.
(e) Properties. Pure titanium has a tensile strength of 100,000 psi; Brinell hardness number of 200; specific gravity of 4.5; melting point of 3300°F (1851°C); and good corrosion resistance. Alloy titanium has a Brinell hardness number of 340; tensile strength of 150,000 psi; and a high strength/weight ratio (twice that of aluminum alloy at 400°F (204°C)).
(f) Appearance test. Titanium is a soft, shiny, silvery-white metal burns in air and is the only element that burns in nitrogen. Titanium alloys look like steel, and can be distinguished from steel by a copper sulfate solution. The solution will not react with titanium, but will leave a coating of copper on steel.
(g) Spark test. The sparks given off are large, brilliant white, and of medium length.
(12) Tungsten (W).
(a) General. Tungsten is a hard, heavy, nonmagnetic metal which will melt at approximately 6150°F (3400°C).
(b) Uses. Tungsten is used in making light bulb filaments, phonograph needles, and as an alloying agent in production of high-speed steel, armorplate, and projectiles. It is also used as an alloying agent in nonconsumable welding electrodes, armor plate, die and tool steels, and hard metal carbide cutting tools.
(c) Capabilities. Tungsten can be cold and hot drawn.
(d) Limitations. Tungsten is hard to machine, requires high temperatures for melting, and is produced by powered metallurgy (sintering process).
(e) Properties. Tungsten has a melting point of 6170 ± 35°F (3410 ± 19°C); is ductile; has tensile strength of 105,000 psi (723,975 kPa); a specific gravity of 19.32; thermal conductivity of 0.397; a Brinell hardness number of 38; and is a dull white color.
(f) Appearance. Tungsten is steel gray in color.
(g) Spark test. Tungsten produces a very small volume of short, straight, orange streaks in a spark test.
(13) Zinc (Zn).
(a) General. Zinc is a medium low strength metal having a very low melting point. Ito is easy to machine, but coarse grain zinc should be heated to approximately 180°F (82°C) to avoid cleavage of crystals. Zinc can be soldered or welded if it is properly cleaned and the heat input closely controlled.
(b) Uses.
1. Galvanizing metal is the largest use of zinc and is done by dipping the part in molten zinc or by electroplating it. Examples of items made in this way are galvanized pipe, tubing, sheet metal, wire, nails, and bolts. Zinc is also used as an alloying element in producing alloys such as brass and bronze. Those alloys that are made up primarily of zinc itself.
2. Typical parts made with zinc alloy are die castings, toys, ornaments, building equipment, carburetor and fuel pump bodies, instrument panels, wet and dry batteries, fuse plugs, pipe organ pipes, munitions, cooking utensils, and flux. Other forms of zinc include zinc oxide and zinc sulfide, widely used in paint and rubber, and zinc dust, which is used in the manufacture of explosives and chemical agents.
(c) Capabilities. Zinc can be cast, cold worked (extruded), machined, and welded.
(d) Limitations. Do not use zinc die castings in continuous contact with steam.
(e) Properties. Zinc has a tensile strength of 12,000 psi (82,740 kPa) (cast) and 27,000 psi (186,165 kPa) (rolled); a specific gravity of 7.1; a melting point of 790°F (421°C); is corrosion resistant; and is brittle at 220°F (104°C).
(f) Appearance. Both zinc and zinc alloys are blue-white in color when polished, and oxidize to gray.
(g) Fracture test. Zinc fractures appear somewhat granular.
(h) Spark test. Zinc and zinc alloys give off no sparks in a spark test.
(i) Zinc die castings.
1. Appearance test. Die castings are usually alloys of zinc, aluminum, magnesium, lead, and tin. They are light in weight, generally silvery white in color (like aluminum), and sometimes of intricate design. A die-cast surface is much smoother than that of a casting made in sand, and is almost as smooth as a machined surface. Sometimes, die castings darkened by use may be mistaken for malleable iron when judged simply by looks, but the die casting is lighter in weight and softer.
2. Fracture test. The surface of a zinc die casting is white and has a slight granular structure.
3. Spark test. Zinc die castings give off no sparks.
4. Torch test. Zinc die castings can be recognized by their low melting temperatures. The metal boils when heated with the oxyacetylene flame. A die casting, after thorough cleaning, can be welded with a carburizing flame using tin or aluminum solders as filler metal. If necessary, the die-cast part can be used as a pattern to make a new brass casting.
(14) White metal die castings.
(a) General. These are usually made with alloys of aluminum, lead, magnesium, or tin. Except for those made of lead and tin, they are generally light in weight and white in color.
(b) Appearance. The surface is much smoother than that produced by castings made in sand.
(c) Fracture test. Fractured surface is white and somewhat granular.
(d) Spark test. No sparks given off in a spark test.
(e) Torch test. Melting points are low, and the metal boils under the torch.
Section II. STANDARD METAL DESIGNATIONS
7-4. GENERAL
The numerical index system for the classification of metals and their alloys has been generally adopted by industry for use on drawings and specifications. In this system, the class to which the metal belongs, the predominant alloying agent, and the average carbon content percentage are given.
7-5. STANDARD DESIGNATION SYSTEM FOR STEEL
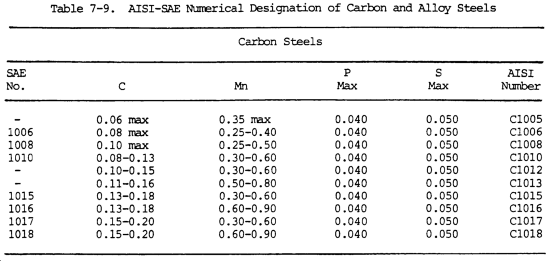
a. Numbers are used to designate different chemical compositions. A four-digit number series designates carbon and alloying steels according to the types and classes shown in table 7-8. This system has been expanded, and in some cases five digits are used to designate certain alloy steels.
b. Two letters are often used as a prefix to the numerals. The letter C indicates basic open hearth carbon steels, and E indicates electric furnace carbon and alloy steels. The letter H is sometimes used as a suffix to denote steels manufactured to meet hardenability limits.
c. The first two digits indicate the major alloying metals in a steel, such as manganese, nickel-chromium, and chrome-molybdenum.
d. The last digits indicate the approximate middle of the carbon content range in percent. For example, 0.21 indicates a range of 0.18 to 0.23 percent carbon. In a few cases, the system deviates from this rule, and some carbon ranges relate to the ranges of manganese, sulfur, phosphorous, chromium, and other elements.
e. The system designates the major elements of a steel and the approximate carbon range of the steel. It also indicates the manufacturing process used to produce the steel. The complete designation system is shown in table 7-9.
f. The number 2340 by this system indicates a nickel steel with approximately 3 percent nickel and 0.40 percent carbon. The number 4340 indicates a nickel-chrome-molybdenum metal with 0.40 percent carbon.
S. A. E. Steel Specifications
The following numerical system for identifying carbon and alloy steels of various specifications has been adopted by the Society of Automotive Engineers.
COMPARISION
A.I.S.I.--S.A.E. Steel Specifications
The ever-growing variety of chemical compositions and quality requirements of steel specifications have resulted in several thousand different combinations of chemical elements being specified to meet individual demands of purchasers of steel products.
The S.A.E. developed a system of nomenclature for identification of various chemical compositions which symbolize certain standards as to machining, heat treating, and carburizing performance. The American Iron and Steel Institute has now gone further in this regard with a new standardization setup with similar nomenclature, but with restricted carbon ranges and combinations of other elements which have been accepted as standard by all manufacturers of bar steel in the steel industry. The Society of Automotive Engineers have, as a result, revised most of their specifications to coincide with those set up by the American Iron and Steel Institute.
PREFIX LETTERS
No prefix for basin open-hearth alloy steel.
(B) Indicates acid Bessemer carbon steel.
(C) Indicates basic open-hearth carbon steel
(E) Indicates electric furnace steel.
NUMBER DESIGNATIONS
(10XX series) Basic open-hearth and acid Bessemer carbon steel grades, non-sulfurized and non-phosphorized.
(11XX series) Basic open-hearth and acid Bessemer carbon steel grades, sulfurized but not phosphorized.
(1300 series) Manganese 1.60 to 1.90%
(23XX series) Nickel 3.50%
(25XX series) Nickel 5.0%
(31XX series) Nickel 1.25%-chromium 0.60%
(33XX series) Nickel 3.50%-chromium 1.60%
(40XX series) Molybdenum
(41XX series) Chromium molybdenum
(43XX series) Nickel-chromium-molybdenum
(46XX series) Nickel 1.65%-molybdenum 0.25%
(48XX series) Nickel 3.25%-molybdenum 0.25%
(51XX series) Chromium
(52XX series) Chromium and high carbon
(61XX series) Chromium vanadium
(86XX series) Chrome nickel molybdenum
(87XX series) Chrome nickel molybdenum
(92XX series) Silicon 2.0%-chromium
(93XX series) Nickel 3.0%-chromium-molybdenum
(94XX series) Nickel-chromium-molybdenum
(97XX series) Nickel-chromium-molybdenum
(98XX series) Nickel-chromium-molybdenum
7-6. STANDARD DESIGNATION SYSTEM FOR ALUMINUM AND ALUMINUM ALLOYS
a. Currently, there is no standard designation system for aluminum castings. Wrought aluminum and aluminum alloys have a standard four-digit numbering system.
b. The first digit represents the major alloying element.
c. The second digit identifies alloy modifications (a zero means the original alloy).
d. The last two digits seine only to identify different aluminum alloys which are in common commercial use, except in the 1XXX class. In the 1XXX class, the last two digits indicate the aluminum content above 99 percent, in hundredths of one percent.
e. In number 1017, the 1 indicates a minimum aluminum composition of 99 percent; the 0 indicates it is the original composition; and the 17 indicates the hundredths of one percent of aluminum above the 99 percent minimum composition. In this example, the aluminum content is 99.17 percent.
f. In number 3217, the 3 indicates a manganese aluminum alloy; the 2 indicates the second modification of this particular alloy; and the 17 indicates a commonly used commercial alloy.
g. The various classes of aluminum and aluminum alloys are identified by numbers as shown in table 7-10.
7-7. STANDARD DESIGNATION SYSTEM FOR MAGNESIUM AND MAGNESIUM ALLOYS
a. Wrought magnesium and magnesium alloys are identified by a combination of letters and numbers. The letters identify which alloying elements were used in the magnesium alloy (table 7-11). Numbers, which may follow the letters, designate the percentage of the elements in the magnesium alloy. There may be an additional letter following the percentage designators which indicates the alloy modifications. For example, the letter A means 1; B means 2; and C means 3.
b. In the identification number AZ93C, the A indicates aluminum; the Z indicates zinc; the 9 indicates there is 9 percent aluminum in the alloy; the 3 indicates there is 3 percent zinc in the alloy; and the C indicates the third modification to the alloy. The first digit, 9 in this example, always indicates the percentage of the first letter, A in this example. The second digit gives the percentage of the second letter (table 7-12).
c. Temper designations may be added to the basic magnesium designation, the two being separated by a dash. The temper designations are the same as those used for aluminum (see Heat Treatment of Steel in Chapter 12).
7-8. STANDARD DESIGNATION SYSTEM FOR COPPER AND COPPER ALLOYS
a. There are over 300 different wrought copper and copper alloys commercially available. The Copper Development Association, Inc., has established an alloy designation system that is widely accepted in North America. It is not a specification system but rather a method of identifying and grouping different coppers and copper alloys. This system has been updated so that it now fits the unified numbering system (UNS). It provides one unified numbering ring system which includes all of the commercially available metals and alloys. The UNS designation consists of the prefix letter C followed by a space, three digits, another space, and, finally, two zeros.
b. The information shown by table 7-13 is a grouping of these copper alloys by common names which normally include the constituent alloys. Welding information for those alloy groupings is provided. There may be those alloys within a grouping that may have a composition sufficiently different to create welding problems. These are the exception, however, and the data presented will provide starting point guidelines. There are two categories, wrought materials and cast materials. The welding information is the same whether the material is cast or rolled.
7-9. STANDARD DESIGNATION SYSTEM FOR TITANIUM
There is no recognized standard designation system for titanium and titanium alloys. However, these compositions are generally designated by using the chemical symbol for titanium, Ti, followed by the percentage number(s) and the chemical symbols(s) of the alloying element(s). For example, Ti-5 A1-2.5 Sn would indicate that 5 percent aluminum and 2-1/2 percent tin alloying elements are present in the titanium metal.
Section III. GENERAL DESCRIPTION AND WELDABILITY OF FERROUS METALS
7-10. LOW CARBON STEELS
a. General. The low carbon (mild) steels include those with a carbon content of up to 0.30 percent (fig. 7-7). In most low carbon steels, carbon ranges from 0.10 to 0.25 percent, manganese from 0.25 to 0.50 percent, phosphorous 0.40 percent maximum, and sulfur 0.50 percent maximum. Steels in this range are most widely used for industrial fabrication and construction. These low carbon steels do not harden appreciably when welded, and therefore do not require preheating or postheating except in special cases, such as when heavy sections are to be welded. In general, no difficulties are encountered when welding low carbon steels. Properly made low carbon steel welds will equal or exceed the base metal in strength. Low carbon steels are soft, ductile, can be rolled, punched, sheared, and worked when either hot or cold. They can be machined and are readily welded. Cast steel has a rough, dark gray surface except where machined. Rolled steel has fine surface lines running in one direction. Forged steel is usually recognizable by its shape, hammer marks, or fins. The fracture color is bright crystalline gray, and the spark test yields sparks with long, yellow-orange streaks that have a tendency to burst into white, forked sparklers. Steel gives off sparks when melted and solidifies almost instantly. Low carbon steels can be easily welded with any of the arc, gas, and resistance welding processes.
b. Copper coated low carbon rods should be used for welding low carbon steel. The rod sizes for various plate thicknesses are as follows:
|
Plate thickness |
Rod diameter |
NOTE
Rods from 5/16 to 3/8 in. (7.9 to 9.5 mm) are available for heavy welding. However, heavy welds can be made with the 3/16 or 1/4 in. (4.8 or 6.4 mm) rods by properly controlling the puddle and melting rate of the rod.
c. The joints may be prepared by flame cutting or machining. The type of preparation (fig. 7-8) is determined by the plate thickness and the welding position.
d. The flame should be adjusted to neutral. Either the forehand or backhand welding method may be used (para 6-23 through 6-24), depending on the thickness of the plates being welded.
e. The molten metal should not be overheated, because this will cause the metal to boil and spark excessively. The resultant grain structure of the weld metal will be large, the strength lowered, and the weld badly scarred.
f. The low carbon steels do not harden in the fusion zone as a result of welding.
g. Metal-Arc Welding.
(1) When metal-arc welding low carbon steels, the bare, thin coated or heavy coated shielded arc types of electrodes may be used. These electrodes are of low carbon type (0.10 to 0.14 percent).
(2) Low carbon sheet or plate materials that have been exposed to low temperatures should be preheated slightly to room temperature before welding.
(3) In welding sheet metal up to 1/8 in. (3.2 mm) in thickness, the plain square butt joint type of edge preparation may be used. When long seams are to be welded in these materials, the edges should be spaced to allow for shrinkage, because the deposited metal tends to pull the plates together. This shrinkage is less severe in arc welding than in gas welding, and spacing of approximately 1/8 in. (3.2 mm) will be sufficient.
(4) The backstep, or skip, welding technique should be used for short seams that are fixed in place. This will prevent warpage or distortion, and will minimize residual stresses.
(5) Heavy plates should be beveled to provide an included angle of up to 60 degrees, depending on the thickness. The parts should be tack welded in place at short intervals along the seam. The first, or root, bead should be made with an electrode small enough in diameter to obtain good penetration and fusion at the base of the joint. A 1/8 or 5/32 in. (3.2 or 4.0 mm) electrode is suitable for this purpose. The first bead should be thoroughly cleaned by chipping and wire brushing before additional layers of weld metal are deposited. Additional passes of the filler metal should be made with a 5/32 or 3/16 in. (4.0 or 4.8 mm) electrode. The passes should be made with a weaving motion for flat, horizontal, or vertical positions. When overhead welding, the best results are obtained by using string beads throughout the weld.
(6) When welding heavy sections that have been beveled from both sides, the weave beads should be deposited alternately on one side and then the other. This will reduce the amount of distortion in the welded structure. Each bead should be cleaned thoroughly to remove all scale, oxides, and slag before additional metal is deposited. The motion of the electrode should be controlled so as to make the bead uniform in thickness and to prevent undercutting and overlap at the edges of the weld. All slag and oxides must be removed from the surface of the completed weld to prevent rusting.
h. Carbon-Arc Welding. Low carbon sheet and plate up to 3/4 in. (19.0 mm) in thickness can be welded using the carbon-arc welding process. The arc is struck against the plate edges, which are prepared in a manner similar to that required for metal-arc welding. A flux should be used on the joint and filler metal should be added as in oxyacetylene welding. A gaseous shield should be provided around the molten base. Filler metal, by means of a flux coated welding rod, should also be provided. Welding must be done without overheating the molten metal. Failure to observe these precautions can cause the weld metal to absorb an excessive amount of carbon from the electrode and oxygen and nitrogen from the air, and cause brittleness in the welded joint.
7-11. MEDIUM CARBON STEELS
a. General. Medium carbon steels are non-alloy steels which contain from 0.30 to 0.55 percent carbon. These steels may be heat treated after fabrication and used for general machining and forging of parts which require surface hardness and strength. They are manufactured in bar form and in the cold rolled or the normalized and annealed condition. When heat treated steels are welded, they should be preheated from 300 to 500°F (149 to 260°C), depending on the carbon content (0.25 to 0.45 percent) and the thickness of the steel. The preheating temperature may be checked by applying a stick of 50-50 solder (melting point 450°F (232°C)) to the plate at the joint, and noting when the solder begins to melt. During welding, the weld zone will become hardened if cooled rapidly, and must be stress relieved after welding. Medium carbon steels may be welded with any of the arc, gas, and resistance welding processes.
b. With higher carbon and manganese content, the low-hydrogen type electrodes should be used, particularly in thicker sections. Electrodes of the low-carbon, heavy coated, straight or reverse polarity type, similar to those used for metal-arc welding of low carbon steels, are satisfactory for welding medium carbon steels.
c. Small parts should be annealed to induce softness before welding. The parts should be preheated at the joint and welded with a filler rod that produces heat treatable welds. After welding, the entire piece should be heat treated to restore its original properties.
d. Either a low carbon or high strength rod can be used for welding medium carbon steels. The welding flame should be adjusted to slightly carburizing, and the puddle of metal kept as small as possible to make a sound joint. Welding with a carburizing flame causes the metal to heat quickly, because heat is given off when steel absorbs carbon. This permits welding at higher speeds.
e. Care should be taken to slowly cool the parts after welding to prevent cracking of the weld. The entire welded part should be stress relieved by heating to between 1100 and 1250°F (593 and 677°C) for one hour per inch (25.4 mm) of thickness, and then slowly cooling. Cooling can be accomplished by covering the parts with fire resistant material or sand.
f. Medium carbon steels can be brazed by using a preheat of 200 to 400°F (93 to 204°C), a good bronze rod, and a brazing flux. However, these steels are better welded by the metal-arc process with mild steel shielded arc electrodes.
g. When welding mild steels, keep the following general techniques in mind:
(1) The plates should be prepared for welding in a manner similar to that used for welding low carbon steels. When welding with low carbon steel electrodes, the welding heat should be carefully controlled to avoid overheating the weld metal and excessive penetration into the side walls of the joint. This control is accomplished by directing the electrode more toward the previously deposited filler metal adjacent to the side walls than toward the side walls directly. By using this procedure, the weld metal is caused to wash up against the side of the joint and fuse with it without deep or excessive penetration.
(2) High welding heats will cause large areas of the base metal in the fusion zone adjacent to the welds to become hard and brittle. The area of these hard zones in the base metal can be kept to a minimum by making the weld with a series of small string or weave beads, which will limit the heat input. Each bead or layer of weld metal will refine the grain in the weld immediately beneath it, and will anneal and lessen the hardness produced in the base metal by the previous bead.
(3) When possible, the finished joint should be heat treated after welding. Stress relieving is normally used when joining mild steel, and high carbon alloys should be annealed.
(4) In welding medium carbon steels with stainless steel electrodes, the metal should be deposited in string beads in order to prevent cracking of the weld metal in the fusion zone. When depositing weld metal in the upper layers of welds made on heavy sections, the weaving motion of the electrode should not exceed three electrode diameters.
(5) Each successive bead of weld should be chipped, brushed, and cleaned prior to the laying of another bead.
7-12. HIGH CARBON STEELS
a. General. High carbon steels include those with a carbon content exceeding 0.55 percent. The unfinished surface of high carbon steels is dark gray and similar to other steels. High carbon steels usually produce a very fine grained fracture, whiter than low carbon steels. Tool steel is harder and more brittle than plate steel or other low carbon material. High carbon steel can be hardened by heating to a good red and quenching in water. Low carbon steel, wrought iron, and steel castings cannot be hardened. Molten high carbon steel is brighter than low carbon steel, and the melting surface has a cellular appearance. It sparks more freely than low carbon (mild) steel, and the sparks are whiter. These steels are used to manufacture tools which are heat treated after fabrication to develop the hard structure necessary to withstand high shear stress and wear. They are manufactured in bar, sheet, and wire forms, and in the annealed or normalized and annealed condition in order to be suitable for machining before heat treatment. The high carbon steels are difficult to weld because of the hardening effect of heat at the welded joint. Because of the high carbon content and the heat treatment usually given to these steels, their basic properties are impaired by arc welding.
b. The welding heat changes the properties of high carbon steel in the vicinity of the weld. To restore the original properties, heat treatment is necessary.
c. High carbon steels should be preheated from 500 to 800°F (260 to 427°C) before welding. The preheating temperature can be checked with a pine stick, which will char at these temperatures.
d. Since high carbon steels melt at lower temperatures than low and medium carbon steels, care should be taken not to overheat the weld or base metal. Overheating is indicated by excessive sparking of the molten metal. Welding should be completed as soon as possible and the amount of sparking should be used as a check on the welding heat. The flame should be adjusted to carburizing. This type of flame tends to produce sound welds.
e. Either a medium or high carbon welding rod should be used to make the weld. After welding, the entire piece should be stress relieved by heating to between 1200 and 1450°F (649 and 788°C) for one hour per inch (25.4 mm) of thickness, and then slowly cooling. If the parts can easily be softened before welding, a high carbon welding rod should be used to make the joint. The entire piece should then be heat treated to restore the original properties of the base metal.
f. In some cases, minor repairs to these steels can be made by brazing. This process does not require temperatures as high as those used for welding, so the properties of the base metal are not seriously affected. Brazing should only be used in special cases, because the strength of the joint is not as high as the original base metal.
g. Either mild or stainless steel electrodes can be used with high carbon steels.
h. Metal-arc welding in high carbon steels requires critical control of the weld heat. The following techniques should be kept in mind:
(1) The welding heat should be adjusted to provide good fusion at the side walls and root of the joint without excessive penetration. Control of the welding heat can be accomplished by depositing the weld metal in small string beads. Excessive puddling of the metal should be avoided, because this can cause carbon to be picked up from the base metal, which in turn will make the weld metal hard and brittle. Fusion between the filler metal and the side walls should be confined to a narrow zone. Use the surface fusion procedure prescribed for medium carbon steels (para 7-11).
(2) The same procedure for edge preparation, cleaning of the welds, and sequence of welding beads as prescribed for low and medium carbon steels also applies to high carbon steels.
(3) Small, high carbon steel parts are sometimes repaired by building up worn surfaces. When this is done, the piece should be annealed or softened by heating to a red heat and cooling slowly. The piece should then be welded or built up with medium carbon or high strength electrodes, and heat treated after welding to restore its original properties.
7-13. TOOL STEELS
a. General. Steels used for making tools, punches, and dies are perhaps the hardest, strongest, and toughest steels used in industry. In general, tool steels are medium to high carbon steels with specific elements included in different amounts to provide special characteristics. A spark test shows a moderately large volume of white sparks having many fine, repeating bursts.
b. Carbon is provided in tool steel to help harden the steel for cutting and wear resistance. Other elements are added to provide greater toughness or strength. In some cases, elements are added to retain the size and shape of the tool during its heat treat hardening operation, or to make the hardening operation safer and to provide red hardness so that the tool retains its hardness and strength when it becomes extremely hot. Iron is the predominant element in the composition of tool steels. Other elements added include chromium, cobalt, manganese, molybdenum, nickel, tungsten, and vanadium. The tool or die steels are designed for special purposes that are dependent upon composition. Certain tool steels are made for producing die blocks; some are made for producing molds, others for hot working, and others for high-speed cutting application.
c. Another way to classify tool steels is according to the type of quench required to harden the steel. The most severe quench after heating is the water quench (water-hardening steels). A less severe quench is the oil quench, obtained by cooling the tool steel in oil baths (oil-hardening steels). The least drastic quench is cooling in air (air-hardening steels).
d. Tool steels and dies can also be classified according to the work that is to be done by the tool. This is based on class numbers.
(1) Class I steels are used to make tools that work by a shearing or cutting actions, such as cutoff dies, shearing dies, blanking dies, and trimming dies.
(2) Class II steels are used to make tools that produce the desired shape of the part by causing the material being worked, either hot or cold, to flow under tension. This includes drawing dies, forming dies, reducing dies, forging dies, plastic molds, and die cast molding dies.
(3) Class III steels are used to make tools that act upon the material being worked by partially or wholly reforming it without changing the actual dimensions. This includes bending dies, folding dies, and twisting dies.
(4) Class IV steels are used to make dies that work under heavy pressure and that produce a flow of metal or other material caressing it into the desired form. This includes crimping dies, embossing dies, heading dies, extrusion dies, and staking dies.
e. Steels in the tool steels group have a carbon content ranging from 0.83 to 1.55 percent. They are rarely welded by arc welding because of the excessive hardness produced in the fusion zone of the base metal. If arc welding must be done, either mild steel or stainless steel electrodes can be used.
f. Uniformly high preheating temperatures (up to 1000°F (583°C)) must be used when welding tool steels.
g. In general, the same precautions should be taken as those required for welding high carbon steels (para 6-12). The welding flare should be adjusted to carburizing to prevent the burning out of carbon in the weld metal. The welding should be done as quickly as possible, taking care not to overheat the molten metal. After welding, the steel should be heat treated to restore its original properties.
h. Drill rods can be used as filler rods because their high carbon content compares closely with that of tool steels.
i. A flux suitable for welding cast iron should be used in small quantities to protect the puddle of high carbon steel and to remove oxides in the weld metal.
j. Welding Technique. When welding tool steels, the following techniques should be kept in mind:
(1) If the parts to be welded are small, they should be annealed or softened before welding. The edges should then be preheated up to 1000°F (538°C), depending on the carbon content and thickness of the plate. Welding should be done with either a mild steel or high strength electrode.
(2) High carbon electrodes should not be used for welding tool steels. The carbon picked up from the base metal by the filler metal will cause the weld to become glass hard, whereas the mild steel weld metal can absorb additional carbon without becoming excessively hard. The welded part should then be heat treated to restore its original properties.
(3) When welding with stainless steel electrodes, the edge of the plate should be preheated to prevent the formation of hard zones in the base metal. The weld metal should be deposited in small string beads to keep the heat input to a minimum. In general, the application procedure is the same as that required for medium and high carbon steels.
k. There are four types of die steels that are weld repairable. These are water-hardening dies, oil-hardening dies, air-hardening dies, and hot work tools. High-speed tools can also be repaired.
7-14. HIGH HARDNESS ALLOY STEELSa. General. A large number and variety of obtain high strength, high hardness, corrosion alloy steels have been developed to resistance, and other special properties. Most of these steels depend on a special heat treatment process in order to develop the desired characteristic in the finished state. Alloy steels have greater strength and durability than other carbon steels, and a given strength is secured with less material weight.
b. High hardness alloy steels include the following:
(1) Chromium alloy steels. Chromium is used as an alloying element in carbon steels to increase hardenability, corrosion resistance, and shock resistance, and gives high strength with little loss in ductility. Chromium in large amounts shortens the spark stream to one half that of the same steel without chromium, but does not affect the stream�s brightness.
(2) Nickel alloy steels. Nickel increases the toughness, strength, and ductility of steels, and lowers the hardening temperature so that an oil quench, rather than a water quench, is used for hardening. The nickel spark has a short, sharply defined dash of brilliant light just before the fork.
(3) High chromium-nickel alloy (stainless) steels. These high alloy steels cover a wide range of compositions. Their stainless, corrosion, and heat resistant properties vary with the alloy content, and are due to the formation of a very thin oxide film which forms on the surface of the metal. Sparks are straw colored near the grinding wheel, and white near the end of the streak. There is a medium volume of streaks which have a moderate number of forked bursts.
(4) Manganese alloy steels. Manganese is used in steel to produce greater toughness, wear resistance, easier hot rolling, and forging. An increase in manganese content decreases the weldability of steel. Steels containing manganese produce a spark similar to a carbon spark. A moderate increase in manganese increases the volume of the spark stream and the intensity of the bursts. A steel containing more than a normal amount of manganese will produce a spark similar to a high carbon steel with a lower manganese content.
(5) Molybdenum alloy steels. Molybdenum increases hardenability, which is the depth of hardening possible through heat treatment. The impact fatigue property of the steel is improved with up to 0.60 percent molybdenum. Above 0.60 percent molybdenum, the impact fatigue proper is impaired. Wear resistance is improved with molybdenum content above about 0.75 percent. Molybdenum is sometimes combined with chromium, tungsten, or vanadium to obtain desired properties. Steels containing this element produce a characteristic spark with a detached arrowhead similar to that of wrought iron, which can be seen even in fairly strong carbon bursts. Molybdenum alloy steels contain either nickel and/or chromium.
(6) Titanium and columbium (niobium) alloy steels. These elements are used as additional alloying agents in low carbon content, corrosion resistant steels. They support resistance to intergranular corrosion after the metal is subjected to high temperatures for a prolonged period of time.
(7) Tungsten alloy steels. Tungsten, as an alloying element in tool steel, tends to produce a fine, dense grain when used in relatively small quantities. When used in larger quantities, from 17 to 20 percent, and in combination with other alloys, tungsten produces a steel that retains its hardness at high temperatures. This element is usually used in combination with chromium or other alloying agents. In a spark test, tungsten will show a dull red color in the spark stream near the wheel. It also shortens the spark stream and decreases the size of or completely eliminates the carbon burst. A tungsten steel containing about 10 percent tungsten causes short, curved, orange spear points at the end of the carrier lines. Still lower tungsten content causes small, white bursts to appear at the end of the spear petit. Carrier lines may be from dull red to orange, depending on the other elements present, providing the tungsten content is not too high.
(8) Vanadium alloy steels. Vanadium is used to help control grain size. It tends to increase hardenability and causes marked secondary hardness, yet resists tempering. It is added to steel during manufacture to remove oxygen. Alloy steels containing vanadium produce sparks with detached arrowheads at the end of the carrier line similar to those produced by molybdenum steels.
(9) Silicon alloy steels. Silicon is added to steel to obtain greater hardenability and corrosion resistance. It is often used with manganese to obtain a strong, tough steel.
(10) High speed tool steels. These steels are usually special alloy compositions designed for cutting tools. The carbon content ranges from 0.70 to 0.80 percent. They are difficult to weld, except by the furnace induction method. A spark test will show a few long, forked spades which are red near the wheel, and straw colored near the end of the spark stream.
c. Many of these steels can be welded with a heavy coated electrode of the shielded arc type, whose composition is similar to that of the base metal. Low carbon electrodes can also be used with some steels. Stainless steel electrodes are effective where preheating is not feasible or desirable. Heat treated steels should be preheated, if possible, in order to minimize the formation of hard zones, or layers, in the base metal adjacent to the weld. The molten metal should not be overheated, and the welding heat should be controlled by depositing the metal in narrow string beads. In many cases, the procedures for welding medium carbon steels (para 7-11) and high carbon steels (para 7-12) can be used in the welding of alloy steels.
7-15. HIGH YIELD STRENGTH, LOW ALLOY STRUCTURAL STEELS
a. General. High yield strength, low alloy structural steels (constructional alloy steels) are special steels that are tempered to obtain extreme toughness and durability. The special alloys and general makeup of these steels require special treatment to obtain satisfactory weldments. These steels are special, low-carbon steels containing specific, small amounts of alloying elements. They are quenched and tempered to obtain a yield strength of 90,000 to 100,000 psi (620,550 to 689,500 kPa) and a tensile strength of 100,000 to 140,000 psi (689,500 to 965,300 kPa), depending upon size and shape. Structural members fabricated from these high strength steels may have smaller cross-sectional areas than common structural steels and still have equal strength. These steels are also more corrosion and abrasion resistant than other steels. In a spark test, these alloys produce a spark very similar to low carbon steels.
b. Welding Technique. Reliable welding of high yield strength, low alloy structural steels can be performed by using the following guidelines:
CAUTION
To prevent underbead cracking, only low hydrogen electrodes should be used when welding high yield strength, low alloy structural steels.
(1) Correct electrodes. Hydrogen is the number one enemy of sound welds in alloy steels; therefore, use only low hydrogen (MIL-E-18038 or MIL-E-22200/1) electrodes to prevent underbead cracking. Underbead cracking is caused by hydrogen picked up in the electrode coating, released into the arc, and absorbed by the molten metal.
(2) Moisture control of electrodes. If the electrodes are in an airtight container, place them, immediately upon opening the container, in a ventilated holding oven set at 250 to 300°F (121 to 149°C). In the event that the electrodes are not in an airtight container, put them in a ventilated baking oven and bake for 1-1/4 hours at 800°F (427°C). Baked electrodes should, while still warm, be placed in the holding oven until used. Electrodes must be kept dry to eliminate absorption of hydrogen. Testing for moisture should be in accordance with MIL-E-22200.
NOTE
Moisture stabilizer NSN 3439-00-400-0090 is an ideal holding oven for field use (MIL-M-45558).
c. Low Hydrogen Electrode Selection. Electrodes are identified by classification numbers which are always marked on the electrode containers. For low hydrogen coatings, the last two nunbers of the classification should be 15, 16, or 18. Electrodes of 5/32 and 1/8 in. (4.0 and 3.2 mm) in diameter are the most commonly used, since they are more adaptable to all types of welding of this type steel. Table 7-14 lists electrodes used to weld high yield strength, low alloy structural steels. Table 7-15 is a list of electrodes currently established in the Army supply system.
d. Selecting Wire-Flux and Wire-Gas Combinations. Wire electrodes for submerged arc and gas-shielded arc welding are not classified according to strength. Welding wire and wire-flux combinations used for steels to be stress relieved should contain no more than 0.05 recent vanadium. Weld metal with more than 0.05 percent vanadium may brittle if stress relieved. When using either the submerged arc or gas metal-arc welding processes to weld high yield strength, low alloy structural steels to lower strength steels the wire-flux and wire-gas combination should be the same as that recommended for the lower strength steels.
e. Preheating. For welding plates under 1.0 in. (25.4 mm) thick, above 50°F (10°C) is not required except to remove surface moisture metal. Table 7-16 contains suggested preheating temperatures.
f. Welding Heat.
(1) General. It is important to avoid excessive heat concentration in order to allow the weld area to cool quickly. Either the heat input nomograph or the heat input calculator can be used to determine the heat input into the weld.
(2) Heat input nomograph. To use the heat input nomograph (fig. 7-9), find the volts value in column 1 and draw a line to the amps value in column 3. From the point where this line intersects colunm 2, draw another line to the in./min value in column 5. Read the heat units at the point where this second line intersects column 4. The heat units represent thousands of joules per inch. For example, at 20 volts and 300 amps, the line intersects column 2 at the value 6. At 12 in./min, the heat input is determined as 30 heat units, or 30,000 joules/in.
(3) Heat input calculator. The heat input calculator can be made by copying the pattern printed on the inside of the back cover of this manual onto plastic, light cardboard, or other suitable material and cutting out the pieces. If no suitable material is available, the calculator may be assembled by cutting the pattern out of the back cover. After the two pieces are cut out, a hole is punched in the center of each. They are then assembled using a paper fastener, or some similar device, which will allow the pieces to rotate. To determine welding heat input using the calculator, rotate until the value on the volts scale is aligned directly opposite the value on the speed (in./min) scale. The value on the amps scale will then be aligned directly opposite the calculated value for heat units. As with the nomograph, heat units represent thousands of joules per inch.
(4) Maximum heat input. Check the heat input value obtained from the nomograph or calculator against the suggested maximums in tables 7-17 and 7-18. If the calculated value is too high, adjust the amperes, travel speed, or preheat temperature until the calculated heat input is within the proper range. (The tables are applicable only to single-arc, shielded metal-arc, submerged arc, gas tungsten-arc, flux-cored arc, and gas metal-arc processes. They are not applicable to multiple-arc or electroslag welding, or other high heat input vertical-welding processes, since welds made by these in the "T-1" steels should be heat treated by quenching and tempering.) For welding conditions exceeding the range of the nomograph or calculator, the heat input can be calculated using the following formula:

g. Welding Process. Reliable welding of high yield strength, low alloy structural steel can be per formal by choosing an electrode with low hydrogen content or selecting the proper wire-flux or wire gas combination when using the submerged arc or gas metal arc processes. Use a straight stringer bead whenever possible. Avoid using the weave pattern; however, if needed, it must be restricted to a partial weave pattern. Best results are obtained by a slight circular motion of the electrode with the weave area never exceeding two elect-rode diameters. Never use a full weave pattern. The partial weave pattern should not exceed twice the diameter of the electrode. Skip weld as practical. Peening of the weld is sometimes recommended to relieve stresses while cooling larger pieces. Fillet welds should be smooth and correctly contoured. Avoid toe cracks and undercutting. Electrodes used for fillet welds should be of lower strength than those used for butt welding. Air-hammer peening of fillet welds can help to prevent cracks, especially if the welds are to be stress relieved. A soft steel wire pedestal can help to absorb shrinkage forces. Butter welding in the toe area before actual fillet welding strengths the area where a toe crack may start. A bead is laid in the toe area, then ground off prior to the actual fillet welding. This butter weld bead must be located so that the toe of the fillet will be laid directly over it during actual fillet welding. Because of the additional material involved in fillet welding, the cooling rate is increased and heat inputs may be extended about 25 percent.
7-16. CAST IRON
a. General. A cast iron is an alloy of iron, carbon, and silicon, in which the amount of carbon is usually more than 1.7 percent and less than 4.5 percent.
(1) The most widely used type of cast iron is known as gray iron. Gray iron has a variety of compositions, but is usually such that it is primarily perlite with many graphite flakes dispersed throughout.
(2) There are also alloy cast irons which contain small amounts of chromium, nickel, molybdenum, copper, or other elements added to provide specific properties.
(3) Another alloy iron is austenitic cast iron, which is modified by additions of nickel and other elements to reduce the transformation temperature so that the structure is austenitic at room or normal temperatures. Austenitic cast irons have a high degree of corrosion resistance.
(4) In white cast iron, almost all the carbon is in the combined form. This provides a cast iron with higher hardness, which is used for abrasion resistance.
(5) Malleable cast iron is made by giving white cast iron a special annealing heat treatment to change the structure of the carbon in the iron. The structure is changed to perlitic or ferritic, which increases its ductility.
(6) Nodular iron and ductile cast iron are made by the addition of magnesium or aluminum which will either tie up the carbon in a combined state or will give the free carbon a spherical or nodular shape, rather than the normal flake shape in gray cast iron. This structure provides a greater degree of ductility or malleability of the casting.
(7) Cast irons are widely used in agricultural equipment; on machine tools as bases, brackets, and covers; for pipe fittings and cast iron pipe; and for automobile engine blocks, heads, manifolds, and water preps. Cast iron is rarely used in structural work except for compression members. It is widely used in construction machinery for counterweights and in other applications for which weight is required.
b. Gray cast iron has low ductility and therefore will not expand or stretch to any considerable extent before breaking or cracking. Because of this characteristic, preheating is necessary when cast iron is welded by the oxyacetylene welding process. It can, however, be welded with the metal-arc process without preheating if the welding heat is carefully controlled. This can be accomplished by welding only short lengths of the joint at a time and allowing these sections to cool. By this procedure, the heat of welding is confined to a small area, and the danger of cracking the casting is eliminated. Large castings with complicated sections, such as motor blocks, can be welded without dismantling or preheating. Special electrodes designed for this purpose are usually desirable. Ductile cast irons, such as malleable iron, ductile iron, and nodular iron, can be successfully welded. For best results, these types of cast irons should be welded in the annealed condition.
c. Welding is used to salvage new iron castings, to repair castings that have failed in service, and to join castings to each other or to steel parts in manufacturing operations. Table 7-19 shows the welding processes that can be used for welding cast, malleable, and nodular irons. The selection of the welding process and the welding filler metals depends on the type of weld properties desired and the service life that is expected. For example, when using the shielded metal arc welding process, different types of filler metal can be used. The filler metal will have an effect on the color match of the weld compared to the base material. The color match can be a determining factor, specifically in the salvage or repair of castings, where a difference of color would not be acceptable.
d. No matter which of the welding processes is selected, certain preparatory steps should be made. It is important to determine the exact type of cast iron to be welded, whether it is gray cast iron or a malleable or ductile type. If exact information is not known, it is best to assume that it is gray cast iron with little or no ductility. In general, it is not recommended to weld repair gray iron castings that are subject to heating and cooling in normal service, especially when heating and cooling vary over a range of temperatures exceeding 400°F (204°C). Unless cast iron is used as the filler material, the weld metal and base metal may have different coefficients of expansion and contraction. This will contribute to internal stresses which cannot be withstood by gray cast iron. Repair of these types of castings can be made, but the reliability and service life on such repairs cannot be predicted with accuracy.
e. Preparation for Welding.
(1) In preparing the casting for welding, it is necessary to remove all surface materials to completely clean the casting in the area of the weld. This means removing paint, grease, oil, and other foreign material from the weld zone. It is desirable to heat the weld area for a short time to remove entrapped gas from the weld zone of the base metal. The skin or high silicon surface should also be removed adjacent to the weld area on both the face and root side. The edges of a joint should be chipped out or ground to form a 60° angle or bevel. Where grooves are involved, a V groove from a 60-90° included angle should be used. The V should extend approximately 1/8 in. (3.2 mm) from the bottom of the crack. A small hole should be drilled at each end of the crack to keep it from spreading. Complete penetration welds should always be used, since a crack or defect not completely removed may quickly reappear under service conditions.
(2) Preheating is desirable for welding cast irons with any of the welding processes. It can be reduced when using extremely ductile filler metal. Preheating will reduce the thermal gradient between the weld and the remainder of the cast iron. Preheat temperatures should be related to the welding process, the filler metal type, the mass, and the complexity of the casting. Preheating can be done by any of the normal methods. Torch heating is normally used for relatively small castings weighing 30.0 lb (13.6 kg) or less. Larger parts may be furnace preheated, and in some cases, temporary furnaces are built around the part rather than taking the part to a furnace. In this way, the parts can be maintained at a high interpass temperature in the temporary furnace during welding. Preheating should be general, since it helps to improve the ductility of the material and will spread shrinkage stresses over a large area to avoid critical stresses at any one point. Preheating tends to help soften the area adjacent to the weld; it assists in degassing the casting, and this in turn reduces the possibility of porosity of the deposited weld metal; and it increases welding speed.
(3) Slow cooling or post heating improves the machinability of the heat-affected zone in the cast iron adjacent to the weld. The post cooling should be as slow as possible. This can be done by covering the casting with insulating materials to keep the air or breezes from it.
f. Welding Technique.
(1) Electrodes.
(a) Cast iron can be welded with a coated steel electrode, but this method should be used as an emergency measure only. When using a steel electrode, the contraction of the steel weld metal, the carbon picked up from the cast iron by the weld metal, and the hardness of the weld metal caused by rapid cooling must be considered. Steel shrinks more than cast iron when ceded from a molten to a solid state. When a steel electrode is used, this uneven shrinkage will cause strains at the joint after welding. When a large quantity of filler metal is applied to the joint, the cast iron may crack just back of the line of fusion unless preventive steps are taken. To overcome these difficulties, the prepared joint should be welded by depositing the weld metal in short string beads, 0.75 to 1.0 in. long (19.0 to 25.4 mm). These are made intermittently and, in some cases, by the backstep and skip procedure. To avoid hard spots, the arc should be struck in the V, and not on the surface of the base metal. Each short length of weld metal applied to the joint should be lightly peened while hot with a small ball peen hammer, and allowed to cool before additional weld metal is applied. The peening action forges the metal and relieves the cooling strains.
(b) The electrodes used should be 1/8 in. (3.2 mm) in diameter to prevent excessive welding heat. Welding should be done with reverse polarity. Weaving of the electrode should be held to a minimum. Each weld metal deposit should be thoroughly cleaned before additional metal is added.
(c) Cast iron electrodes must be used where subsequent machining of the welded joint is required. Stainless steel electrodes are used when machining of the weld is not required. The procedure for making welds with these electrodes is the same as that outlined for welding with mild steel electrodes. Stainless steel electrodes provide excellent fusion between the filler and base metals. Great care must be taken to avoid cracking in the weld, contracts approximately 50 percent more than because stainless steel expands and mild steel in equal changes of temperature.
(2) Arc Welding.
(a) The shielded metal arc welding process can be utilized for welding cast iron. There are four types of filler metals that may be used: cast iron covered electrodes; covered copper base alloy electrodes; covered nickel base alloy electrodes; and mild steel covered electrodes. There are reasons for using each of the different specific types of electrodes, which include the machinability of the deposit, the color match of the deposit, the strength of the deposit, and the ductility of the final weld.
(b) When arc welding with the cast iron electrodes (ECI), preheat to between 250 and 800°F (121 and 425°C), depending on the size and complexity of the casting and the need to machine the deposit and adjacent areas. The higher degree of heating, the easier it will be to machine the weld deposit. In general, it is best to use small-size electrodes and a relatively 1ow current setting. A medium arc length should be used, and, if at all possible, welding should be done in the flat position. Wandering or skip welding procedure should be used, and peening will help reduce stresses and will minimize distortion. Slow cooling after welding is recommended. These electrodes provide an excellent color match cm gray iron. The strength of the weld will equal the strength of the base metal. There are two types of copper-base electrodes: the copper tin alloy and the copper aluminum types. The copper zinc alloys cannot be used for arc welding electrodes because of the low boiling temperature of zinc. Zinc will volatilize in the arc and will cause weld metal porosity.
(c) When the copper base electrodes are used, a preheat of 250 to 400°F (121 to 204°C) is recommended. Small electrodes and low current should be used. The arc should be directed against the deposited metal or puddle to avoid penetration and mixing the base metal with the weld metal. Slow cooling is recommended after welding. The copper-base electrodes do not provide a good color match.
(d) There are three types of nickel electrodes used for welding cast iron. These electrodes can be used without preheat; however, heating to 100°F (38°C) is recommended. These electrodes can be used in all positions; however, the flat position is recommended. The welding slag should be removed between passes. The nickel and nickel iron deposits are extremely ductile and will not become brittle with the carbon pickup. The hardness of the heat-affected zone can be minimized by reducing penetration into the cast iron base metal. The technique mentioned above, playing the arc on the puddle rather than on the base metal, will help minimize dilution. Slow cooling and, if necessary, postheating will improve machinability of the heat-affected zone. The nickel-base electrodes do not provide a close color match.
(e) Copper nickel type electrodes cane in two grades. Either of these electrodes can be used in the same manner as the nickel or nickel iron electrode with about the same technique and results. The deposits of these electrodes do not provide a color match.
(f) Mild steel electrodes are not recommended for welding cast iron if the deposit is to be machined. The mild steel deposit will pick up sufficient carbon to make a high-carbon deposit, which is impossible to machine. Additionally, the mild steel deposit will have a reduced level of ductility as a result of increased carbon content. This type of electrode should be used only for small repairs and should not be used when machining is required. Minimum preheat is possible for small repair jobs. Small electrodes at low current are recommended to minimize dilution and to avoid the concentration of shrinkage stresses. Short welds using a wandering sequence should be used, and the weld should be peened as quickly as possible after welding. The mild steel electrode deposit provides a fair color match.
(3) Carbon-arc welding of cast iron. Iron castings may be welded with a carbon arc, a cast iron rod, and a cast iron welding flux. The joint should be preheated by moving the carbon electrodes along the surface. This prevents too-rapid cooling after welding. The molten puddle of metal can be worked with the carbon electrode so as to move any slag or oxides that are formed to the surface. Welds made with the carbon arc cool more slowly and are not as hard as those made with the metal arc and a cast iron electrode. The welds are machinable.
(4) Oxyfuel gas welding. The oxyfuel gas process is often used for welding cast iron. Most of the fuel gases can be used. The flame should be neutral to slightly reducing. Flux should be used. Two types of filler metals are available: the cast iron rods and the copper zinc rods. Welds made with the proper cast iron electrode will be as strong as the base metal. Good color match is provided by all of these welding reds. The optimum welding procedure should be used with regard to joint preparation, preheat, and post heat. The copper zinc rods produce braze welds. There are two classifications: a manganese bronze and a low-fuming bronze. The deposited bronze has relatively high ductility but will not provide a color match.
(5) Brazing and braze welding.
(a) Brazing is used for joining cast iron to cast iron and steels. In these cases, the joint design must be selected for brazing so that capillary attraction causes the filler metal to flow between closely fitting parts. The torch method is normally used. In addition, the carbon arc, the twin carbon arc, the gas tungsten arc, and the plasma arc can all be used as sources of heat. Two brazing filler metal alloys are normally used; both are copper zinc alloys. Braze welding can also be used to join cast iron. In braze welding, the filler metal is not drawn into the joint by capillary attraction. This is sometimes called bronze welding. The filler material having a liquidous above 850°F (454°C) should be used. Braze welding will not provide a color match.
(b) Braze welding can also be accomplished by the shielded metal arc and the gas metal arc welding processes. High temperature preheating is not usually required for braze welding unless the part is extremely heavy or complex in geometry. The bronze weld metal deposit has extremely high ductility, which compensates for the lack of ductility of the cast iron. The heat of the arc is sufficient to bring the surface of the cast iron up to a temperature at which the copper base filler metal alloy will make a bond to the cast iron. Since there is little or no intermixing of the materials, the zone adjacent to the weld in the base metal is not appreciably hardened. The weld and adjacent area are machinable after the weld is completed. In general, a 200°F (93°C) preheat is sufficient for most application. The cooling rate is not extremely critical and a stress relief heat treatment is not usually required. This type of welding is commonly used for repair welding of automotive parts, agricultural implement parts, and even automotive engine blocks and heads. It can only be used when the absence of color match is not objectionable.
(6) Gas metal arc welding. The gas metal arc welding process can be used for making welds between malleable iron and carbon steels. Several types of electrode wires can be used, including:
(a) Mild steel using 75% argon + 25% CO2 for shielding.
(b) Nickel copper using 100% argon for shielding.
(c) Silicon bronze using 50% argon + 50% helium for shielding.
In all cases, small diameter electrode wire should be used at low current. With the mild steel electrode wire, the Argon-CO2 shielding gas mixture issued to minimize penetration. In the case of the nickel base filler metal and the Copper base filler metal, the deposited filler metal is extremely ductile. The mild steel provides a fair color match. A higher preheat is usually required to reduce residual stresses and cracking tendencies.
(7) Flux-cored arc welding. This process has recently been used for welding cast irons. The more successful application has been using a nickel base flux-cored wire. This electrode wire is normally operated with CO2 shielding gas, but when lower mechanical properties are not objectionable, it can be operated without external shielding gas. The minimum preheat temperatures can be used. The technique should minimize penetration into the cast iron base metal. Postheating is normally not required. A color match is not obtained.
(8) Studding. Cracks in large castings are sometimes repaired by studding (fig. 7-10). In this process, the fracture is removed by grinding a V groove. Holes are drilled and tapped at an angle on each side of the groove, and studs are screwed into these holes for a distance equal to the diameter of the studs, with the upper ends projecting approximately 1/4 in. (6.4 mm) above the cast iron surface. The studs should be seal welded in place by one or two beads around each stud, and then tied together by weld metal beads. Welds should be made in short lengths, and each length peened while hot to prevent high stresses or cracking upon cooling. Each bead should be allowed to cool and be thoroughly cleaned before additional metal is deposited. If the studding method cannot be applied, the edges of the joint should be chipped out or machined with a round-nosed tool to form a U groove into which the weld metal should be deposited.
(9) Other welding processes can be used for cast iron. Thermit welding has been used for repairing certain types of cast iron machine tool parts. Soldering can be used for joining cast iron, and is sometimes used for repairing small defects in small castings. Flash welding can also be used for welding cast iron.
Section IV. GENERAL DESCRIPTION AND WELDABILITY OF NONFERROUS METALS
7-17. ALUMINUM WELDING
a. General. Aluminum is a lightweight, soft, low strength metal which can easily be cast, forged, machined, formed and welded. Unless alloyed with specific elements, it is suitable only in low temperature applications. Aluminum is light gray to silver in color, very bright when polished, and dull when oxidized. A fracture in aluminum sections shows a smooth, bright structure. Aluminum gives off no sparks in a spark test, and does not show red prior to melting. A heavy film of white oxide forms instantly on the molten surface. Its combination of light weight and high strength make aluminum the second most popular metal that is welded. Aluminum and aluminum alloys can be satisfactorily welded by metal-arc, carbon-arc, and other arc welding processes. The principal advantage of using arc welding processes is that a highly concentrated heating zone is obtained with the arc. For this reason, excessive expansion and distortion of the metal are prevented.
b. Alloys. Many alloys of aluminum have been developed. It is important to know which alloy is to be welded. A system of four-digit numbers has been developed by the Aluminum Association, Inc., to designate the various wrought aluminum alloy types. This system of alloy groups, shown by table 7-20, is as follows:
(1) 1XXX series. These are aluminums of 99 percent or higher purity which are used primarily in the electrical and chemical industries.
(2) 2XXX series. Copper is the principal alloy in this group, which provides extremely high strength when properly heat treated. These alloys do not produce as good corrosion resistance and are often clad with pure aluminum or special-alloy aluminum. These alloys are used in the aircraft industry.
(3) 3XXX series. Manganese is the major alloying element in this group, which is non-heat-treatable. Manganese content is limited to about 1.5 percent. These alloys have moderate strength and are easily worked.
(4) 4XXX series. Silicon is the major alloying element in this group. It can be added in sufficient quantities to substantially reduce the melting point and is used for brazing alloys and welding electrodes. Most of the alloys in this group are non-heat-treatable.
(5) 5XXX series. Magnesium is the major alloying element of this group, which are alloys of medium strength. They possess good welding characteristics and good resistance to corrosion, but the amount of cold work should be limited.
(6) 6XXX series. Alloys in this group contain silicon and magnesium, which make them heat treatable. These alloys possess medium strength and good corrosion resistance.
(7) 7XXX series. Zinc is the major alloying element in this group. Magnesium is also included in most of these alloys. Together, they form a heat-treatable alloy of very high strength, which is used for aircraft frames.
c. Welding Aluminum Alloys. Aluminum possesses a number of properties that make welding it different than the welding of steels. These are: aluminum oxide surface coating; high thermal conductivity; high thermal expansion coefficient; low melting temperature; and the absence of color change as temperature approaches the melting point. The normal metallurgical factors that apply to other metals apply to aluminum as well.
(1) Aluminum is an active metal which reacts with oxygen in the air to produce a hard, thin film of aluminum oxide on the surface. The melting point of aluminum oxide is approximately 3600°F (1982°C) which is almost three times the melting point of pure aluminum (1220°F (660°C)). In addition, this aluminum oxide film absorbs moisture from the air, particularly as it becomes thicker. Moisture is a source of hydrogen, which causes porosity in aluminum welds. Hydrogen may also come from oil, paint, and dirt in the weld area. It also comes from the oxide and foreign materials on the electrode or filler wire, as well as from the base metal. Hydrogen will enter the weld pool and is soluble in molten aluminum. As the aluminum solidifies, it will retain much less hydrogen. The hydrogen is rejected during solidification. With a rapid cooling rate, free hydrogen is retained within the weld and will cause porosity. Porosity will decrease weld strength and ductility, depending on the amount.
CAUTION
Aluminum and aluminum alloys should not be cleaned with caustic soda or cleaners with a pH above 10, as they may react chemically.
(a) The aluminum oxide film must be removed prior to welding. If it is not completely removed, small particles of unmelted oxide will be trapped in the weld pool and will cause a reduction in ductility, lack of fusion, and possibly weld cracking.
(b) The aluminum oxide can be removed by mechanical, chemical, or electrical means. Mechanical removal involves scraping with a sharp tool, sandpaper, wire brush (stainless steel), filing, or any other mechanical method. Chemical removal can be done in two ways. One is by use of cleaning solutions, either the etching types or the nonetching types. The nonetching types should be used only when starting with relatively clean parts, and are used in conjunction with other solvent cleaners. For better cleaning, the etching type solutions are recommended, but must be used with care. When dipping is employed, hot and cold rinsing is highly recommended. The etching type solutions are alkaline solutions. The time in the solution must be controlled so that too much etching does not occur.
(c) Chemical cleaning includes the use of welding fluxes. Fluxes are used for gas welding, brazing, and soldering. The coating on covered aluminum electrodes also maintains fluxes for cleaning the base metal. Whenever etch cleaning or flux cleaning is used, the flux and alkaline etching materials must be completely removed from the weld area to avoid future corrosion.
(d) The electrical oxide removal system uses cathodic bombardment. Cathodic bombardment occurs during the half cycle of alternating current gas tungsten arc welding when the electrode is positive (reverse polarity). This is an electrical phenomenon that actually blasts away the oxide coating to produce a clean surface. This is one of the reasons why AC gas tungsten arc welding is so popular for welding aluminum.
(e) Since aluminum is so active chemically, the oxide film will immediately start to reform. The time of buildup is not extremely fast, but welds should be made after aluminum is cleaned within at least 8 hours for quality welding. If a longer time period occurs, the quality of the weld will decrease.
(2) Aluminum has a high thermal conductivity and low melting temperature. It conducts heat three to five times as fast as steel, depending on the specific alloy. More heat must be put into the aluminum, even though the melting temperature of aluminum is less than half that of steel. Because of the high thermal conductivity, preheat is often used for welding thicker sections. If the temperature is too high or the time period is too long, weld joint strength in both heat-treated and work-hardened alloys may be diminished. The preheat for aluminum should not exceed 400°F (204°C), and the parts should not be held at that temperature longer than necessary. Because of the high heat conductivity, procedures should utilize higher speed welding processes using high heat input. Both the gas tungsten arc and the gas metal arc processes supply this requirement. The high heat conductivity of aluminum can be helpful, since the weld will solidify very quickly if heat is conducted away from the weld extremely fast. Along with surface tension, this helps hold the weld metal in position and makes all-position welding with gas tungsten arc and gas metal arc welding practical.
(3) The thermal expansion of aluminum is twice that of steel. In addition, aluminum welds decrease about 6 percent in volume when solidifying from the molten state. This change in dimension may cause distortion and cracking.
(4) The final reason aluminum is different from steels when welding is that it does not exhibit color as it approaches its melting temperature until it is raised above the melting point, at which time it will glow a dull red. When soldering or brazing aluminum with a torch, flux is used. The flux will melt as the temperature of the base metal approaches the temperature required. The flux dries out first, and melts as the base metal reaches the correct working temperature. When torch welding with oxyacetylene or oxyhydrogen, the surface of the base metal will melt first and assume a characteristic wet and shiny appearance. (This aids in knowing when welding temperatures are reached.) When welding with gas tungsten arc or gas metal arc, color is not as important, because the weld is completed before the adjoining area melts.
d. Metal-Arc Welding of Aluminum.
(1) Plate welding. Because of the difficulty of controlling the arc, butt and fillet welds are difficult to produce in plates less than 1/8 in. (3.2 mm) thick. When welding plate heavier than 1/8 in. (3.2 mm), a joint prepared with a 20 degree bevel will have strength equal to a weld made by the oxyacetylene process. This weld may be porous and unsuitable for liquid-or gas-tight joints. Metal-arc welding is, however, particularly suitable for heavy material and is used on plates up to 2-1/2 in. (63.5 mm) thick.
(2) Current and polarity settings. The current and polarity settings will vary with each manufacturer's type of electrodes. The polarity to be used should be determined by trial on the joints to be made.
(3) Plate edge preparation. In general, the design of welded joints for aluminum is quite consistent with that for steel joints. However, because of the higher fluidity of aluminum under the welding arc, some important general principles should be kept in mind. With the lighter gauges of aluminum sheet, less groove spacing is advantageous when weld dilution is not a factor. The controlling factor is joint preparation. A specially designed V groove that is applicable to aluminum is shown in A, figure 7-11. This type of joint is excellent where welding can be done from one side only and where a smooth, penetrating bead is desired. The effectiveness of this particular design depends upon surface tension, and should be applied on all material over 1/8 in. (3.2 mm) thick. The bottom of the special V groove must be wide enough to contain the root pass completely. This requires adding a relatively large amount of filler alloy to fill the groove. Excellent control of the penetration and sound root pass welds are obtained. This edge preparation can be employed for welding in all positions. It eliminates difficulties due to burn-through or over-penetration in the overheat and horizontal welding positions. It is applicable to all weldable base alloys and all filler alloys.
e. Gas Metal-Arc (MIG) Welding (GMAW).
(1) General. This fast, adaptable process is used with direct current re-verse polarity and an inert gas to weld heavier thicknesses of aluminum alloys, in any position, from 1/016 in. (1.6 mm) to several inches thick. TM 5-3431-211-15 describes the operation of a typical MIG welding set.
(2) Shielding gas. Precautions should be taken to ensure the gas shield is extremely efficient. Welding grade argon, helium, or a mixture of these gases is used for aluminum welding. Argon produces a smother and more stable arc than helium. At a specific current and arc length, helium provides deeper penetration and a hotter arc than argon. Arc voltage is higher with helium, and a given change in arc length results in a greater change in arc voltage. The bead profile and penetration pattern of aluminum welds made with argon and helium differ. With argon, the bead profile is narrower and more convex than helium. The penetration pattern shows a deep central section. Helium results in a flatter, wider bead, and has a broader under-bead penetration pattern. A mixture of approximately 75 percent helium and 25 percent argon provides the advantages of both shielding gases with none of the undesirable characteristics of either. Penetration pattern and bead contour show the characteristics of both gases. Arc stability is comparable to argon. The angle of the gun or torch is more critical when welding aluminum with inert shielding gas. A 30° leading travel angle is recommended. The electrode wire tip should be oversize for aluminum. Table 7-21 provides welding procedure schedules for gas metal-arc welding of aluminum.
(3) Welding technique. The electrode wire must be clean. The arc is struck with the electrode wire protruding about 1/2 in. (12.7 mm) from the cup. A frequently used technique is to strike the arc approximately 1.0 in. (25.4 mm) ahead of the beginning of the weld and then quickly bring the arc to the weld starting point, reverse the direction of travel, and proceed with normal welding. Alternatively, the arc may be struck outside the weld groove on a starting tab. When finishing or terminating the weld, a similar practice may be followed by reversing the direction of welding, and simultaneously increasing the speed of welding to taper the width of the molten pool prior to breaking the arc. This helps to avert craters and crater cracking. Runoff tabs are commonly used. Having established the arc, the welder moves the electrode along the joint while maintaining a 70 to 85 degree forehand angle relative to the work. A string bead technique is normally preferred. Care should be taken that the forehand angle is not changed or increased as the end of the weld is approached. Arc travel speed controls the bead size. When welding aluminum with this process, it is must important that high travel speeds be maintained. When welding uniform thicknesses, the electrode to work angle should be equal on both sides of the weld. When welding in the horizontal position, best results are obtained by pointing the gun slightly upward. When welding thick plates to thin plates, it is helpful to direct the arc toward the heavier section. A slight backhand angle is sometimes helpful when welding thin sections to thick sections. The root pass of a joint usually requires a short arc to provide the desired penetration. Slightly longer arcs and higher arc voltages may be used on subsequent passes.
The wire feeding equipment for aluminum welding must be in good adjustment for efficient wire feeding. Use nylon type liners in cable assemblies. Proper drive rolls must be selected for the aluminum wire and for the size of the electrode wire. It is more difficult to push extremely small diameter aluminum wires through long gun cable assemblies than steel wires. For this reason, the spool gun or the newly developed guns which contain a linear feed motor are used for the small diameter electrode wires. Water-cooled guns are required except for low-current welding. Both the constant current (CC) power source with matching voltage sensing wire feeder and the constant voltage (CV) power source with constant speed wire feeder are used for welding aluminum. In addition, the constant speed wire feeder is sometimes used with the constant current power source. In general, the CV system is preferred when welding on thin material and using all diameter electrode wire. It provides better arc starting and regulation. The CC system is preferred when welding thick material using larger electrode wires. The weld quality seems better with this system. The constant current power source with a moderate drop of 15 to 20 volts per 100 amperes and a constant speed wire feeder provide the most stable power input to the weld and the highest weld quality.
(4) Joint design. Edges may be prepared for welding by sawing, machining, rotary planing, routing or arc cutting. Acceptable joint designs are shown in figure 7-12.
f. Gas Tungsten-Arc (TIG) Welding (GTAW).
(1) The gas tungsten arc welding process is used for welding the thinner sections of aluminum and aluminum alloys. There are several precautions that should be mentioned with respect to using this process.
(a) Alternating current is recommended for general-purpose work since it provides the half-cycle of cleaning action. Table 7-22 provides welding procedure schedules for using the process on different thicknesses to produce different welds. AC welding, usually with high frequency, is widely used with manual and automatic applications. Procedures should be followed closely and special attention given to the type of tungsten electrode, size of welding nozzle, gas type, and gas flow rates. When manual welding, the arc length should be kept short and equal to the diameter of the electrode. The tungsten electrode should not protrude too far beyond the end of the nozzle. The tungsten electrode should be kept clean. If it does accidentally touch the molten metal, it must be redressed.
(b) Welding power sources designed for the gas tungsten arc welding process should be used. The newer equipment provides for programming, pre-and post-flow of shielding gas, and pulsing.
(c) For automatic or machine welding, direct current electrode negative (straight polarity) can be used. Cleaning must be extremely efficient, since there is no cathodic bombardment to assist. When dc electrode negative is used, extremely deep penetration and high speeds can be obtained. Table 7-23 lists welding procedure schedules for dc electrode negative welding.
(d) The shielding gases are argon, helium, or a mixture of the two. Argon is used at a lower flow rate. Helium increases penetration, but a higher flow rate is required. When filler wire is used, it must be clean. Oxide not removed from the filler wire may include moisture that will produce polarity in the weld deposit.
(2) Alternating current.
(a) Characteristics of process. The welding of aluminum by the gas tungsten-arc welding process using alternating current produces an oxide cleaning action. Argon shielding gas is used. Better results are obtained when welding aluminum with alternating current by using equipment designed to produce a balanced wave or equal current in both directions. Unbalance will result in loss of power and a reduction in the cleaning action of the arc. Characteristics of a stable arc are the absence of snapping or cracking, smooth arc starting, and attraction of added filler metal to the weld puddle rather than a tendency to repulsion. A stable arc results in fewer tungsten inclusions.
(b) Welding technique. For manual welding of aluminum with ac, the electrode holder is held in one hand and filler rod, if used, in the other. An initial arc is struck on a starting block to heat the electrode. The arc is then broken and reignited in the joint. This technique reduces the tendency for tungsten inclusions at the start of the weld. The arc is held at the starting point until the metal liquefies and a weld pool is established. The establishment and maintenance of a suitable weld pool is important, and welding must not proceed ahead of the puddle. If filler metal is required, it may be added to the front or leading edge of the pool but to one side of the center line. Both hands are moved in unison with a slight backward and forward motion along the joint. The tungsten electrode should not touch the filler rod. The hot end of the filler rod should not be withdrawn from the argon shield. A short arc length must be maintained to obtain sufficient penetration and avoid undercutting, excessive width of the weld bead, and consequent loss of penetration control and weld contour. One rule is to use an arc length approximately equal to the diameter of the tungsten electrode. When the arc is broken, shrinkage cracks may occur in the weld crater, resulting in a defective weld. This defect can be prevented by gradually lengthening the arc while adding filler metal to the crater. Then, quickly break and restrike the arc several times while adding additional filler metal to the crater, or use a foot control to reduce the current at the end of the weld. Tacking before welding is helpful in controlling distortion. Tack welds should be of ample size and strength and should be chipped out or tapered at the ends before welding over.
(c) Joint design. The joint designs shown in figure 7-11 are applicable to the gas tungsten-arc welding process with minor exceptions. Inexperienced welders who cannot maintain a very short arc may require a wider edge preparation, included angle, or joint spacing. Joints may be fused with this process without the addition of filler metal if the base metal alloy also makes a satisfactory filler alloy. Edge and corner welds are rapidly made without addition of filler metal and have a good appearance, but a very close fit is essential.
(3) Direct current straight polarity.
(a) Charcteristics of process. This process, using helium and thoriated tungsten electrodes is advantageous for many automatic welding operations, especially in the welding of heavy sections. Since there is less tendency to heat the electrode, smaller electrodes can be used for a given welding current. This will contribute to keeping the weld bead narrow. The use of direct current straight polarity (dcsp) provides a greater heat input than can be obtained with ac current. Greater heat is developed in the weld pool, which is consequently deeper and narrower.
(b) Welding techniques. A high frequency current should be used to initiate the arc. Touch starting will contaminate the tungsten electrode. It is not necessary to form a puddle as in ac welding, since melting occurs the instant the arc is struck. Care should be taken to strike the arc within the weld area to prevent undesirable marking of the material. Standard techniques such as runoff tabs and foot operated heat controls are used. These are helpful in preventing or filling craters, for adjusting the current as the work heats, and to adjust for a change in section thickness. In dcsp welding, the torch is moved steadily forward. The filler wire is fed evenly into the leading edge of the weld puddle, or laid on the joint and melted as the arc roves forward. In all cases, the crater should be filled to a point above the weld bead to eliminate crater cracks. The fillet size can be controlled by varying filler wire size. DCSP is adaptable to repair work. Preheat is not required even for heavy sections, and the heat affected zone will be smaller with less distortion.
(c) Joint designs. The joint designs shown in figure 7-11 are applicable to the automatic gas tungsten-arc dcsp welding process with minor exceptions. For manual dcsp, the concentrated heat of the arc gives excellent root fusion. Root face can be thicker, grooves narrower, and build up can be easily controlled by varying filler wire size and travel speed.
g. Square Wave Alternating Current Welding (TIG).
(1) General. Square wave gas tungsten-arc welding with alternating current differs frozen conventional balanced wave gas tungsten-arc welding in the type of wave from used. With a square wave, the time of current flow in either direction is adjustable from 20 to 1. In square wave gas tungsten-arc welding, there are the advantages of surface cleaning produced by positive ionic bombardment during the reversed polarity cycle, along with greater weld depth to width ratio produced by the straight polarity cycle. Sufficient aluminum surface cleaning action has been obtained with a setting of approximately 10 percent dcrp. Penetration equal to regular dcsp welding can be obtained with 90 percent dcsp current.
(2) Welding technique. It is necessary to have either superimposed high frequency or high open circuit voltage, because the arc is extinguished every half cycle as the current decays toward zero, and must be restarted each tire. Precision shaped thoriated tungsten electrodes should be used with this process. Argon, helium, or a combination of the two should be used as shielding gas, depending on the application to be used.
(3) Joint design. Square wave alternating current welding offers substantial savings over conventional alternating current balanced wave gas tungsten arc welding in weld joint preparation. Smaller V grooves, U grooves, and a thicker root face can be used. A greater depth to width weld ratio is conducive to less weldment distortion, along with favorable welding residual stress distribution and less use of filler wire. With Some slight modification, the same joint designs can be used as in dcsp gas tungsten-arc welding (fig. 7-11).
h. Shielded Metal-Arc Welding. In the shielded metal-arc welding process, a heavy dipped or extruded flux coated electrode is used with dcrp. The electrodes are covered similarly to conventional steel electrodes. The flux coating provides a gaseous shield around the arc and molten aluminum puddle, and chemically combines and removes the aluminum oxide, forming a slag. When welding aluminum, the process is rather limited due to arc spatter, erratic arc control, limitations on thin material, and the corrosive action of the flux if it is not removed properly.
i. Shielded Carbon-Arc Welding. The shielded carbon-arc welding process can be used in joining aluminum. It requires flux and produces welds of the same appearance, soundness, and structure as those produced by either oxyacetylene or oxyhydrogen welding. Shielded carbon-arc welding is done both manually and automatically. A carbon arc is used as a source of heat while filler metal is supplied from a separate filler rod. Flux must be removed after welding; otherwise severe corrosion will result. Manual shielded carbon-arc welding is usually limited to a thickness of less than 3/8 in. (9.5 mm), accomplished by the same method used for manual carbon arc welding of other material. Joint preparation is similar to that used for gas welding. A flux covered rod is used.
j. Atomic Hydrogen Welding. This welding process consists of maintaining an arc between two tungsten electrodes in an atmosphere of hydrogen gas. The process can be either manual or automatic with procedures and techniques closely related to those used in oxyacetylene welding. Since the hydrogen shield surrounding the base metal excludes oxygen, smaller amounts of flux are required to combine or remove aluminum oxide. Visibility is increased, there are fewer flux inclusions, and a very sound metal is deposited.
k. Stud Welding.
(1) Aluminum stud welding may be accomplished with conventional arc stud welding equipment, using either the capacitor discharge or drawn arc capacitor discharge techniques. The conventional arc stud welding process may be used to weld aluminum studs 3/16 to 3/4 in. (4.7 to 19.0 mm) diameter. The aluminum stud welding gun is modified slightly by the addition of a special adapter for the control of the high purity shielding gases used during the welding cycle. An added accessory control for controlling the plunging of the stud at the completion of the weld cycle adds materially to the quality of weld and reduces spatter loss. Reverse polarity is used, with the electrode gun positive and the workpiece negative. A small cylindrical or cone shaped projection on the end of the aluminum stud initiates the arc and helps establish the longer arc length required for aluminum welding.
(2) The unshielded capacitor discharge or drawn arc capacitor discharge stud welding processes are used with aluminum studs 1/16 to 1/4 in. (1.6 to 6.4 mm) diameter. Capacitor discharge welding uses a low voltage electrostatic storage system, in which the weld energy is stored at a low voltage in capacitors with high capacitance as a power source. In the capacitor discharge stud welding process, a small tip or projection on the end of the stud is used for arc initiation. The drawn arc capacitor discharge stud welding process uses a stud with a pointed or slightly rounded end. It does not require a serrated tip or projection on the end of the stud for arc initiation. In both cases, the weld cycle is similar to the conventional stud welding process. However, use of the projection on the base of the stud provides the most consistent welding. The short arcing time of the capacitor discharge process limits the melting so that shallow penetration of the workpiece results. The minimum aluminum work thickness considered practical is 0.032 in. (0.800 mm).
l. Electron Beam Welding. Electron beam welding is a fusion joining process in which the workpiece is bombarded with a dense stream of high velocity electrons, and virtually all of the kinetic energy of the electrons is transformed into heat upon impact. Electron beam welding usually takes place in an evacuated chamber. The chamber size is the limiting factor on the weldment size. Conventional arc and gas heating melt little more than the surface. Further penetration comes solely by conduction of heat in all directions from this molten surface spot. The fusion zone widens as it depends. The electron beam is capable of such intense local heating that it almost instantly vaporizes a hole through the entire joint thickness. The walls of this hole are molten, and as the hole is moved along the joint, more metal on the advancing side of the hole is melted. This flaws around the bore of the hole and solidifies along the rear side of the hole to make the weld. The intensity of the beam can be diminished to give a partial penetration with the same narrow configuration. Electron beam welding is generally applicable to edge, butt, fillet, melt-thru lap, and spot welds. Filler metal is rarely used except for surfacing.
m. Resistance Welding.
(1) General. The resistance welding processes (spot, seam, and flash welding) are important in fabricating aluminum alloys. These processes are especially useful in joining the high strength heat treatable alloys, which are difficult to join by fusion welding, but can be joined by the resistance welding process with practically no loss in strength. The natural oxide coating on aluminum has a rather high and erratic electrical resistance. To obtain spot or seam welds of the highest strength and consistency, it is usually necessary to reduce this oxide coating prior to welding.
(2) Spot welding. Welds of uniformly high strength and good appearance depend upon a consistently low surface resistance between the workplaces. For most applications, some cleaning operations are necessary before spot or seam welding aluminum. Surface preparation for welding generally consists of removal of grease, oil, dirt, or identification markings, and reduction and improvement of consistency of the oxide film on the aluminum surface. Satisfactory performance of spot welds in service depends to a great extent upon joint design. Spot welds should always be designed to carry shear loads. However, when tension or combined loadings may be expected, special tests should be conducted to determine the actual strength of the joint under service loading. The strength of spot welds in direct tension may vary from 20 to 90 percent of the shear strength.
(3) Seam welding. Seam welding of aluminum and its alloys is very similar to spot welding, except that the electrodes are replaced by wheels. The spots made by a seam welding machine can be overlapped to form a gas or liquid tight joint. By adjusting the timing, the seam welding machine can produce uniformly spaced spot welds equal in quality to those produced on a regular spot welding machine, and at a faster rate. This procedure is called roll spot or intermittent seam welding.
(4) Flash welding. All aluminum alloys may be joined by the flash welding process. This process is particularly adapted to making butt or miter joints between two parts of similar cross section. It has been adapted to joining aluminum to copper in the form of bars and tubing. The joints so produced fail outside of the weld area when tension loads are applied.
n. Gas welding. Gas welding has been done on aluminum using both oxyacetylene and oxyhydrogen flames. In either case, an absolutely neutral flame is required. Flux is used as well as a filler rod. The process also is not too popular because of low heat input and the need to remove flux.
o. Electroslag welding. Electroslag welding is used for joining pure aluminum, but is not successful for welding the aluminum alloys. Submerged arc welding has been used in some countries where inert gas is not available.
p. Other processes. Most of the solid state welding processes, including friction welding, ultrasonic welding, and cold welding are used for aluminums. Aluminum can also be joined by soldering and brazing. Brazing can be accomplished by most brazing methods. A high silicon alloy filler material is used.
7-18. BRASS AND BRONZE WELDING
a. General. Brass and bronze are alloys of copper. Brass has zinc, and bronze has tin as the major alloying elements. However, some bronze metals contain more zinc than tin, and some contain zinc and no tin at all. High brasses contain from 20 to 45 percent zinc. Tensile strength, hardness, and ductility increase as the percentage of zinc increases. These metals are suitable for both hot and cold working.
b. Metal-Arc Welding. Brasses and bronzes can be successfully welded by the metal-arc process. The electrode used should be of the shielded arc type with straight polarity (electrode positive). Brasses can be welded with phosphor bronze, aluminum bronze, or silicon bronze electrodes, depending on the base metal composition and the service required. Backing plates of matching metal or copper should be used. High welding current should not be used for welding copper-zinc alloys (brasses), otherwise the zinc content will be volatilized. All welding should be done in the flat position. If possible, the weld metal should be deposited with a weave approximately three times the width of the electrode.
c. Carbon-Arc Welding. This method can be used to weld brasses and bronzes with filler reds of approximately the same composition as the base metal. In this process, welding is accomplished in much the same way the bronze is bonded to steel. The metal in the carbon arc is superheated, and this very hot metal is alloyed to the base metal in the joint.
d. Oxyacetylene Welding. The low brasses are readily jointed by oxyacetylene welding. This process is particularly suited for piping because it can be done in all welding positions. Silicon copper welding rods or one of the brass welding rods may be used. For oxyacetylene welding of the high brasses, low-fuming welding rods are used. These low-fuming rods have composition similar to many of the high brasses. A flux is required, and the torch flame should be adjusted to a slightly oxidizing flame to assist in controlling fuming. Preheating and an auxiliary heat source may also be necessary. The welding procedures for copper are also suitable for the brasses.
e. Gas Metal Arc Welding. Gas metal arc welding is recommended for joining large phosphor bronze fabrications and thick sections. Direct current, electrode positive, and argon shielding are normally used. The molten weld pool should be kept small and the travel speed rather high. Stringer beads should be used. Hot peening of each layer will reduce welding stresses and the likelihood of cracking.
f. Gas Tungsten Arc Welding. Gas tungsten arc welding is used primarily for repair of castings and joining of phosphor bronze sheet. As with gas metal arc welding, hot peening of each layer of weld metal is beneficial. Either stabilized ac or direct current, electrode negative can be used with helium or argon shielding. The metal should be preheated to the 350 to 400°F (177 to 204°C) range, and the travel speed should be as fast as practical.
g. Shielded Metal Arc Welding. Phosphor bronze covered electrodes are available for joining bronzes of similar compositions. These electrodes are designed for use with direct current, electrode positive. Filler metal should be deposited as stringer beads for best weld joint mechanical properties. Postweld annealing at 900°F (482°C) is not always necessary, but is desirable for maximum ductility, particularly if the weld metal is to be cold worked. Moisture, both on the work and in the electrode coverings, must be strictly avoided. Baking the electrodes at 250 to 300°F (121 to 149°C) before use may be necessary to reduce moisture in the covering to an acceptable level.
7-19. COPPER WELDING
a. General. Copper and copper-base alloys have specific properties which make them widely used. Their high electrical conductivity makes them widely used in the electrical industries, and corrosion resistance of certain alloys makes them very useful in the process industries. Copper alloys are also widely used for friction or bearing applications. Copper can be welded satisfactorily with either bare or coated electrodes. The oxygen free copper can be welded with more uniform results than the oxygen bearing copper, which tends to become brittle when welded. Due to the high thermal conductivity of copper, the welding currents are higher than those required for steel, and preheating of the base metal is necessary. Copper shares some of the characteristics of aluminum, but is weldable. Attention should be given to its properties that make the welding of copper and copper alloys different from the welding of carbon steels. Copper alloys possess properties that require special attention when welding. These are:
(1) High thermal conductivity.
(2) High thermal expansion coefficient.
(3) Relatively low melting point.
(4) Hot short or brittle at elevated temperatures.
(5) Very fluid molten metal.
(6) High electrical conductivity.
(7) Strength due to cold working.
Copper has the highest thermal conductivity of all commercial metals, and the comments made concerning thermal conductivity of aluminum apply to copper, to an even greater degree.
Copper has a relatively high coefficient of thermal expansion, approximately 50 percent higher than carbon steel, but lower than aluminum.
The melting point of the different copper alloys varies over a relatively wide ranger but is at least 1000°F (538°C) lower than carbon steel. Some of the copper alloys are hot short. This means that they become brittle at high temperatures, because some of the alloying elements form oxides and other compounds at the grain boundaries, embrittling the material.
Copper does not exhibit heat colors like steel, and when it melts it is relatively fluid. This is essentially the result of the high preheat normally used for heavier sections. Copper has the highest electrical conductivity of any of the commercial metals. This is a definite problem in the resistance welding processes.
All of the copper alloys derive their strength from cold working. The heat of welding will anneal the copper in the heat-affected area adjacent to the weld, and reduce the strength provided by cold working. This must be considered when welding high-strength joints.
There are three basic groups of copper designations. The first is the oxygen-free type which has a copper analysis of 99.95 percent or higher. The second subgroup are the tough pitch coppers which have a copper composition of 99.88 percent or higher and some high copper alloys which have 96.00 percent or more copper.
The oxygen-free high-conductivity copper contains no oxygen and is not subject to grain boundary migration. Adequate gas coverage should he used to avoid oxygen of the air caning into contact with the molten metal. Welds should be made as quickly as possible, since too much heat or slow welding can contribute to oxidation. The deoxidized coppers are preferred because of their freedom from embrittlement by hydrogen. Hydrogen embrittlement occurs when copper oxide is exposed to a reducing gas at high temperature. The hydrogen reduces the copper oxide to copper and water vapor. The entrapped high temperature water vapor or steam can create sufficient pressure to cause cracking. In common with all copper welding, preheat should be used and can run from 250 to 1000°F (121 to 538°C), depending on the mass involved.
The tough pitch electrolytic copper is difficult to weld because of the presence of copper oxide within the material. During welding, the copper oxide will migrate to the grain boundaries at high temperatures, which reduces ductility and tensile strength. The gas-shielded processes are recommended since the welding area is more localized and the copper oxide is less able to migrate in appreciable quantities.
The third copper subgroup is the high-copper alloys which may contain deoxidizers such as phosphorus. The copper silicon filler wires are used with this material. The preheat temperatures needed to make the weld quickly apply to all three grades.
c. Gas Metal-Arc (MIG) Welding (GMAW).
(1) The gas metal arc welding process is used for welding thicker materials. It is faster, has a higher deposition rate, and usually results in less distortion. It can produce high-quality welds in all positions. It uses direct current, electrode positive. The CV type power source is recommended.
(2) Metal-arc welding of copper differs from steel welding as indicated below:
(a) Greater root openings are required.
(b) Tight joints should be avoided in light sections.
(c) Larger groove angles are required, particularly in heavy sections, in order to avoid excessive undercutting, slag inclusions, and porosity. More frequent tack welds should be used.
(d) Higher preheat and interpass temperatures are required (800°F (427°C) for copper, 700°F (371°C) for beryllium copper).
(e) Higher currents are required for a given size electrode or plate thickness.
(3) Most copper and copper alloy coated electrodes are designed for use with reverse (electrode positive) polarity. Electrodes for use with alternating currents are available.
(4) Peening is used to reduce stresses in the joints. Flat-nosed tools are used for this purpose. Numerous moderate blows should be used, because vigorous blows could cause crystallizations or other defects in the joint.
d. Gas Tungsten-Arc (TIG) Welding (GTAW).
CAUTION
Never use a flux containing fluoride when welding copper or copper alloys.
(1) Copper can be successfully welded by the gas tungsten-arc welding process. The weldability of each copper alloy group by this process depends upon the alloying elements used. For this reason, no one set of welding conditions will cover all groups.
(2) Direct current straight polarity is generally used for welding most copper alloys. However, high frequency alternating current or direct current reverse polarity is used for beryllium copper or copper alloy sheets less than 0.05 in. (0.13 cm) thick.
(3) For some copper alloys, a flux is recommended. However, a flux containing fluoride should never be used since the arc will vaporize the fluoride and irritate the lungs of the operator.
e. Carbon-Arc Welding.
(1) This process for copper welding is most satisfactory for oxygen-free copper, although it can be used for welding oxygen-bearing copper up to 3/8 in. (9.5 mm) in thickness. The root opening for thinner material should be 3/16 in. (4.8 mm), and 3/8 in. (9.5 mm) for heavier material. The electrode should be graphite type carbon, sharpened to a long tapered point at least equal to the size of the welding rod. Phosphor bronze welding rods are used most frequently in this process.
(2) The arc should be sharp and directed entirely on the weld metal, even at the start. If possible, all carbon-arc welding should be done in the flat welding position or on a moderate slope.
7-20. MAGNESIUM WELDING
a. General. Magnesium is a white, very lightweight, machinable, corrosion resistant, high strength metal. It can be alloyed with small quantities of other metals, such as aluminum, manganese, zinc and zirconium, to obtain desired properties. It can be welded by most of the welding processes used in the metal working trades. Because this metal oxidizes rapidly when heated to its melting point in air, a protective inert gas shield must be provided in arc welding to prevent destructive oxidation.
b. Magnesium possesses properties that make welding it different from the welding of steels. Many of these are the same as for aluminum. These are:
(1) Magnesium oxide surface coating which increases with an increase in temperature.
(2) High thermal conductivity.
(3) Relatively high thermal expansion coefficient.
(4) Relatively low melting temperature.
(5) The absence of color change as temperature approaches the melting point.
The normal metallurgical factors that apply to other metals apply to magnesium as well.
c. The welds produced between similar alloys will develop the full strength of the base metals; however, the strength of the heat-affected zone may be reduced slightly. In all magnesium alloys, the solidification range increases and the melting point and the thermal expansion decrease as the alloy content increases. Aluminum added as an alloy up to 10 percent improves weldability, since it tends to refine the weld grain structure. Zinc of more than 1 percent increases hot shortness, which can result in weld cracking. The high zinc alloys are not recommended for arc welding because of their cracking tendencies. Magnesium, containing small amounts of thorium, possesses excellent welding qualities and freedom from cracking Weldments of these alloys do not require stress relieving. Certain magnesium alloys are subject to stress corrosion. Weldments subjected to corrosive attack over a period of time may crack adjacent to welds if the residual stresses are not removed. Stress relieving is required for weldments intended for this type of service.
d. Cleaning. An oil coating or chrome pickle finish is usually provided on magnesium alloys for surface protection during shipment and storage. This oil, along with other foreign matter and metallic oxides, must be removed from the surface prior to welding. Chemical cleaning is preferred, because it is faster and more uniform in its action. Mechanical cleaning can be utilized if chemical cleaning facilities are not available. A final bright chrome pickle finish is recommended for parts that are to be arc welded. The various methods for cleaning magnesium are described below.
WARNING
The vapors from some chlorinated solvents (e.g., carbon tetrachloride, trichloroethylene, and perchloroethylene) break down under the ultraviolet radiation of an electric arc and form a toxic gas. Avoid welding where such vapors are present. These solvents vaporize easily, and prolonged inhalation of the vapor can be hazardous. These organic vapors should be removed from the work area before welding begins.Dry cleaning solvent and mineral spirits paint thinner are highly flammable. Do not clean parts near an open flame or in a smoking area. Dry cleaning solvent and mineral spirits paint thinner evaporate quickly and have a defatting effect on the skin. When used without protective gloves, these chemicals may cause irritation or cracking of the skin. Cleaning operations should be performed only in well ventilated areas.
(2) Mechanical cleaning can be done satisfactorily with 160 and 240 grit aluminum oxide abrasive cloth, stainless steel wool, or by wire brushing.
WARNING
Precleaning and postcleaning acids used in magnesium welding and brazing are highly toxic and corrosive. Goggles, rubber gloves, and rubber aprons should be worn when handling the acids and solutions. Do not inhale fumes and mists. When spilled on the body or clothing, wash immediately with large quantities of cold water, and seek medical attention. Never pour water into acid when preparing solution; instead, pour acid into water. Always mix acid and water slowly. Cleaning operations should be performed only in well ventilated areas.
(3) Immediately after the grease, oil, and other foreign materials have been removed from the surface, the metal should be dipped for 3 minutes in a hot solution with the following composition:

The bath should be operated at 70°F (21°C). The work should be removed from the solution, thoroughly rinsed with hot water, and air dried. The welding rod should also be cleaned to obtain the best results.
e. Joint Preparation. Edges that are to be welded must be smooth and free of loose pieces and cavities that might contain contaminating agents, such as oil or oxides. Joint preparations for arc welding various gauges of magnesium are shown in figure 7-13.
f. Safety Precautions.
CAUTION
Magnesium can ignite and burn when heated in the open atmosphere.
(1) Goggles, gloves, and other equipment designed to protect the eyes and skin of the welder must be worn.
(2) The possibility of fire caused by welding magnesium metal is very remote. The temperature of initial fusion must be reached before solid magnesium metal ignites. Sustained burning occurs only if this temperature is maintained. Finely divided magnesium particles such as grinding dust, filings, shavings, borings, and chips present a fire hazard. They ignite readily if proper precautions are not taken. Magnesium scrap of this type is not common to welding operations. If a magnesium fire does start, it can be extinguished with dry sand, dry powdered soapstone, or dry cast iron chips. The preferred extinguishing agents for magnesium fires are graphite base powders.
g. Gas Tungsten-Arc (TIG) Welding (GTAW) of Magnesium.
(1) Because of its rapid oxidation when magnesium is heated to its melting point, an inert gas (argon or helium) is used to shield metal during arc welding. This process requires no flux and permits high welding speeds, with sound welds of high strength.
(2) Direct current machines of the constant current type operating on straight polarity (electrode positive) and alternating current machines are used with a high frequency current superimposed on the welding current. Both alternating and direct current machines are used for thin gauge material. However, because of better penetrating power, alternating current machines are used on material over 3/16 in. (4.8 mm) thick. Helium is considered more practical than argon for use with direct current reverse polarity. However, three times as much helium by volume as argon is required for a given amount of welding. Argon is used with alternating current.
(3) The tungsten electrodes are held in a water cooled torch equipped with required electrical cables and an inlet and nozzle for the inert gas.
(4) The two magnesium alloys, in the form of sheet, plate, and extrusion, that are most commonly used for applications involving welding are ASTM-1A (Fed Spec QQ-M-54), which is alloyed with manganese, and ASTM-AZ31A (Fed SPec QQ-44), which is alloyed with aluminum, manganese, and zinc.
(5) In general, less preparation is required for welding with alternating current than welding with direct current because of the greater penetration obtained. Sheets up to 1/4 in. (6.4 mm) thickness may be welded from one side with a square butt joint. Sheets over 1/4 in. (6.4 mm) thickness should be welded from both sides whenever the nature of the structure permits, as sounder welds may be obtained with less warpages. For a double V joint, the included angle should extend from both sides to leave a minimum 1/16 in. (1.6 mm) root face in the center of the sheets. When welding a double V joint, the back of the first bead should be chipped out using a chipping hammer fitted with a cape chisel. Remove oxide film, dirt, and incompletely fused areas before the second bead is added. In this manner, maximum soundness is obtained.
(6) The gas should start flowing a fraction of a second before the arc is struck. The arc is struck by brushing the electrode over the surface. With alternating current, the arc should be started and stopped by means of a remote control switch. The average arc length should be about 1/8 in. (3.2 mm) when using helium and 1/16 in. (1.6 mm) when using argon. Current data and rod diameter are shown in table 7-24.
(7) When welding with alternating current, maximum penetration is obtained when the end of the electrode is held flush with or slightly below the surface of the work. The torch should be held nearly perpendicular to the surface of the work, and the welding rod added from a position as neatly parallel with the work as possible (fig. 7-14). The torch should have a slightly leading travel angle.
(8) Welding should progress in a straight line at a uniform speed. There should be no rotary or weaving motion of the rod or torch, except for larger corner joints or fillet welds. The welding rod can be fed either continuously or intermittently. Care should be taken to avoid withdrawing the heated end from the protective gaseous atmosphere during the welding operation. The cold wire filler metal should be brought in as near to horizontal as possible (on flat work). The filler wire is added to the leading edge of the weld puddle. Runoff tabs are recommended for welding any except the thinner metals. Forehand welding, in which the welding rod precedes the torch in the direction of welding, is preferred. If stops are necessary, the weld should be started about 1/2 in. (12.7 mm) back from the end of the weld when welding is resumed.
(9) Because of the high coefficient of thermal expansion and conductivity, control of distortion in the welding of magnesium requires jigging, small beads, and a properly selected welding sequence to help minimize distortion. Magnesium parts can be straightened by holding them in position with clamps and heating to 300 to 400°F (149 to 204°C). If this heating is done by local torch application, care must be taken not to overheat the metal and destroy its properties.
(10) If cracking is encountered during the welding of certain magnesium alloys, starting and stopping plates may be used to overcome this difficult. These plates consist of scrap pieces of magnesium stock butted against opposite ends of the joint to be welded as shown in A, figure 7-15. The weld is started on one of the abutting plates, continued across the junction along the joint to be welded, and stopped on the opposite abutting plate. If a V groove is used, the abutting plates should also be grooved. An alternate method is to start the weld in the middle of the joint and weld to each edge (B, fig. 7-15). Cracking may also be minimized by preheating the plate and holding the jig to 200 to 400°F (93 to 204°C) by increasing the speed of the weld.
(11) Filler reds must be of the same composition as the alloy being joined when arc welding. One exception is when welding AZ31B. In this case, grade C rod (MIL-R-6944), which produces a stronger weld metal, is used to reduce cracking.
(12) Residual stress should be relieved through heat treatment. Stress relief is essential so that lockup stresses will not cause stress corrosion cracking. The recommended stress relieving treatment for arc welding magnesium sheet is shown in table 7-25.
(13) The only cleaning required after arc welding of magnesium alloys is wire brushing to remove the slight oxide deposit on the surface. Brushing may leave traces of iron, which may cause galvanic corrosion. If necessary, clean as in b above. Arc welding smoke can be removed by immersing the parts for 1/2 to 2 minutes at 180 to 212°F (82 to 100°C), in a solution composed of 16 oz (453 g) tetrasodium pyrophosphate (Na4P2O7), 16 oz (453 g) sodium metaborate (NaBO2), and enough water to make 1 gallon (3.8 1).
(14) Welding procedure schedules for GTAW of magnesium (TIG welding) are shown in table 7-26.
h. Gas Metal-Arc (MIG) Welding of Magnesium (GMAW). The gas metal arc welding process is used for the medium to thicker sections. It is considerably faster than gas tungsten arc welding. Special high-speed gear ratios are usually required in the wire feeders since the magnesium electrode wire has an extremely high meltoff rate. The normal wire feeder and power supply used for aluminum welding is suitable for welding magnesium. Different types of arc transfer can be obtained when welding magnesium. This is primarily a matter of current level or current density and voltage setting. The short-circuiting transfer and the spray transfer are recommended. Argon is usually used for gas metal arc welding of magnesium; however, argon-helium mixtures can be used. In general, the spray transfer should be used on material 3/16 in. (4.8 mm) and thicker and the short-circuiting arc used for thinner metals. Welding procedure schedules for GMAW of magnesium (MIG welding) are shown in table 7-27.
i. Other Welding Processes. Magnesium can be welded using the resistance welding processes, including spot welding, seam welding, and flash welding. Magnesium can also be joined by brazing. Most of the different brazing techniques can be used. In all cases, brazing flux is required and the flux residue must be completely removed from the finish part. Soldering is not as effective, since the strength of the joint is relatively low. Magnesium can also be stud welded, gas welded, and plasma-arc welded.
7-21. TITANIUM WELDING
a. General.
(1) Titanium is a soft, silvery white, medium strength metal with very good corrosion resistance. It has a high strength to weight ratio, and its tensile strength increases as the temperature decreases. Titanium has low impact and creep strengths. It has seizing tendencies at temperatures above 800°F (427°C).
(2) Titanium has a high affinity for oxygen and other gases at elevated temperatures, and for this reason, cannot be welded with any process that utilizes fluxes, or where heated metal is exposed to the atmosphere. Minor amounts of impurities cause titanium to become brittle.
(3) Titanium has the characteristic known as the ductile-brittle transition. This refers to a temperature at which the metal breaks in a brittle manner, rather than in a ductile fashion. The recrystallization of the metal during welding can raise the transition temperature. Contamination during the high temperate period and impurities can raise the transition temperature period and impurities can raise the transition temperature so that the material is brittle at room temperatures. If contamination occurs so that transition temperature is raised sufficiently, it will make the welding worthless. Gas contamination can occur at temperatures below the melting point of the metal. These temperatures range from 700°F (371°C) up to 1000°F (538°C).
(4) At room temperature, titanium has an impervious oxide coating that resists further reaction with air. The oxide coating melts at temperatures considerably higher than the melting point of the base metal and creates problems. The oxidized coating may enter molten weld metal and create discontinuities which greatly reduce the strength and ductility of the weld.
(5) The procedures for welding titanium and titanium alloys are similar to other metals. Some processes, such as oxyacetylene or arc welding processes using active gases, cannot be used due to the high chemical activity of titanium and its sensitivity to embrittlement by contamination. Processes that are satisfactory for welding titanium and titanium alloys include gas shielded metal-arc welding, gas tungsten arc welding, and spot, seam, flash, and pressure welding. Special procedures must be employed when using the gas shielded welding processes. These special procedures include the use of large gas nozzles and trailing shields to shield the face of the weld from air. Backing bars that provide inert gas to shield the back of the welds from air are also used. Not only the molten weld metal, but the material heated above 1000°F (538°C) by the weld must be adequately shielded in order to prevent embrittlement. All of these processes provide for shielding of the molten weld metal and heat affected zones. Prior to welding, titanium and its alloys must be free of all scale and other material that might cause weld contamination.
b. Surface Preparation.
WARNING
The nitric acid used to preclean titanium for inert gas shielded arc welding is highly toxic and corrosive. Goggles, rubber gloves, and rubber aprons must be worn when handling acid and acid solutions. Do not inhale gases and mists. When spilled on the body or clothing, wash immediately with large quantities of cold water, and seek medical help. Never pour water into acid when preparing the solution; instead, pour acid into water. Always mix acid and water slowly. Perform cleaning operations only in well ventilated places.The caustic chemicals (including sodium hydride) used to preclean titanium for inert gas shielded arc welding are highly toxic and corrosive. Goggles, rubber gloves, and rubber aprons must be worn when handling these chemicals. Do not inhale gases or mists. When spilled on the body or clothing, wash immediately with large quantities of cold water and seek medical help. Special care should be taken at all times to prevent any water from coming in contact with the molten bath or any other large amount of sodium hydride, as this will cause the formation of highly explosive hydrogen gas.
(1) Surface cleaning is important in preparing titanium and its alloys for welding. Proper surface cleaning prior to welding reduces contamination of the weld due to surface scale or other foreign materials. Small amounts of contamination can render titanium completely brittle.
(2) Several cleaning procedures are used, depending on the surface condition of the base and filler metals. Surface conditions most often encountered are as follows:
(a) Scale free (as received from the mill).
(b) Light scale (after hot forming or annealing at intermediate temperature; ie., less than 1300°F (704°C).
(c) Heavy scale (after hot forming, annealing, or forging at high temperature).
(3) Metals that are scale free can be cleaned by simple decreasing.
(4) Metals with light oxide scale should be cleaned by acid pickling. In order to minimize hydrogen pickup, pickling solutions for this operation should have a nitric acid concentration greater than 20 percent. Metals to be welded should be pickled for 1 to 20 minutes at a bath temperature from 80 to 160°F (27 to 71°C). After pickling, the parts are rinsed in hot water.
(5) Metals with a heavy scale should be cleaned with sand, grit, or vaporblasting, molten sodium hydride salt baths, or molten caustic baths. Sand, grit, or vaporblasting is preferred where applicable. Hydrogen pickup may occur with molten bath treatments, but it can be minimized by controlling the bath temperature and pickling time. Bath temperature should be held at about 750 to 850°F (399 to 454°C). Parts should not be pickled any longer than necessary to remove scale. After heavy scale is removed, the metal should be pickled as described in (4) above.
(6) Surfaces of metals that have undergone oxyacetylene flame cutting operations have a very heavy scale, and may contain microscopic cracks due to excessive contamination of the metallurgical characteristics of the alloys. The best cleaning method for flame cut surfaces is to remove the contaminated layer and any cracks by machining operations. Certain alloys can be stress relieved immediately after cutting to prevent the propagation of these cracks. This stress relief is usually made in conjunction with the cutting operation.
c. MIG or TIG Welding of titanium.
(1) General. Both the MIG and TIG welding processes are used to weld titanium and titanium alloys. They are satisfactory for manual and automatic installations. With these processes, contamination of the molten weld metals and adjacent heated zones is minimized by shielding the arc and the root of the weld with inert gases (see (2)(b)) or special backing bars (see (2)(c)). In some cases, inert gas filler welding chambers (see (3)) are used to provide the required shielding. When using the TIG welding process, a thoriated tungsten electrode should be used. The electrode size should be the smallest diameter that will carry the welding current. The electrode should be ground to a point. The electrode may extend 1-1/2 times its diameter beyond the end of the nozzle. Welding is done with direct current, electrode negative (straight polarity). Welding procedure for TIG welding titanium are shown in table 7-28. Selection of the filler metal will depend upon the titanium alloys being joined. When welding pure titanium, a pure titanium wire should be used. When welding a titanium alloy, the next lowest strength alloy should be used as a filler wire. Due to the dilution which will take place dining welding, the weld deposit will pick up the required strength. The same considerations are true when MIG welding titanium.
(2) Shielding.
(a) General. Very good shielding conditions are necessary to produce arc welded joints with maximum ductility and toughness. To obtain these conditions, the amount of air or other active gases which contact the molten weld metals and. adjacent heated zones must be very low. Argon is normally used with the gas-shielded process. For thicker metal, use helium or a mixture of argon and helium. Welding grade shielding gases are generally free from contamination; however, tests can be made before welding. A simple test is to make a bead on a piece of clean scrap titanium, and notice its color. The bead should be shiny. Any discoloration of the surface indicates a contamination. Extra gas shielding provides protection for the heated solid metal next to the weld metal. This shielding is provided by special trailing gas nozzles, or by chill bars laid immediately next to the weld. Backup gas shielding should be provided to protect the underside of the weld joint. Protection of the back side of the joint can also be provided by placing chill bars in intimate contact with the backing strips. If the contact is close enough, backup shielding gas is not required. For critical applications, use an inert gas welding chamber. These can be flexible, rigid, or vacuum-purge chambers.
(b) Inert gases. Both helium and argon are used as the shielding gases. With helium as the shielding gas high welding speeds and better penetration are obtained than with argon, but the arc is more stable in argon. For open air welding operations, most welders prefer argon as the shielding gas because its density is greater than that of air. Mixtures of argon and helium are also used. With mixtures, the arc characteristics of both helium and argon are obtained. The mixtures usually vary in composition from about 20 to 80 percent argon. They are often used with the consumable electrode process. To provide adequate shielding for the face and root sides of welds, special precautions often are taken. The precautions include the use of screens and baffles (see (c) 3), trailing shields (see (c) 7), and special backing fixtures (see (c) 10) in open air welding, and the use of inert gas filler welding chambers.
(c) Open air welding.
1. In open air welding operations, the methods used to shield the face of the weld vary with joint design, welding conditions, and the thickness of the materials being joined. The most critical area in regard to the shielding is the molten weld puddle. Impurities diffuse into the molten metal very rapidly and remain in solution. The gas flowing through a standard welding torch is sufficient to shield the molten zone. Because of the low thermal conductivity of titanium, however, the molten puddle tends to be larger than most metals. For this reason and because of shielding conditions required in welding titanium, larger nozzles are used on the welding torch, with proportionally higher gas flows that are required for other metals. Chill bars often are used to limit the size of the puddle.
2. The primary sources of contamination in the molten weld puddle are turbulence in the gas flow, oxidation of hot filler reds, insufficient gas flow, small nozzles on the welding torch, and impure shielding gases. The latter three sources are easily controlled.
3. If turbulence occurs in the gas flowing from the torch, air will be inspired and contamination will result. Turbulence is generally caused by excessive amounts of gas flowing through the torch, long arc lengths, air currents blowing across the weld, and joint design. Contamination from this source can be minimized by adjusting gas flows and arc lengths, and by placing baffles alongside the welds. Baffles protect the weld from drafts and tend to retard the flow of shielding gas from the joint area. Chill bars or the clamping toes of the welding jig can serve as baffles (fig. 7-16). Baffles are especially important for making corner type welds. Additional precautions can be taken to protect the operation from drafts and turbulence. This can be achieved by erecting a canvas (or other suitable material) screen around the work area.
4. In manual welding operations with the tungsten-arc process, oxidation of the hot filler metal is a very important source of contamination. To control it, the hot end of the filler wire must be kept within the gas shield of the welding torch. Welding operators must be trained to keep the filler wire shielded when welding titanium and its alloys. Even with proper manipulation, however, contamination from this source probably cannot be eliminated completely.
5. Weld contamination which occurs in the molten weld puddle is especially hazardous. The impurities go into solution, and do not cause discoloration. Although discolored welds may have been improperly shielded while molten, weld discoloration is usually caused by contamination which occurs after the weld has solidified.
6. Most of the auxiliary equipment used on torches to weld titanium is designed to improve shielding conditions for the welds as they solidify and cool. However, if the welding heat input is low and the weld cools to temperatures below about 1200 to 1300°F (649 to 704°C) while shielded, auxiliary shielding equipment is not required. If the weld is at an excessively high temperature after it is no longer shielded by the welding torch, auxiliary shielding must be supplied.
7. Trailing shields often are used to supply auxiliary shielding. These shields extend behind the welding torch and vary considerably in size, shaper and design. They are incorporated into special cups which are used on the welding torch, or may consist only of tubes or hoses attached to the torch or manipulated by hand to direct a stream of inert gas on the welds. Figure 7-17 shows a drawing of one type of trailing shield currently in use. Important features of this shield are that the porous diffusion plate allows an even flow of gas over the shielded area. This will prevent turbulence in the gas stream. The shield fits on the torch so that a continuous gas stream between the torch and shield is obtained.
8. Baffles are also beneficial in improving shielding conditions for welds by retarding the flow of shielding gas from the joint area. Baffles may be placed alongside the weld, over the top, or at the ends of the weld. In some instances, they may actually form a chamber around the arc and molten weld puddle. Also, chill bars may be used to increase weld cooling rates and may make auxiliary shielding unnecessary.
9. Very little difficulty has been encountered in shielding the face of welds in automatic welding operations. However, considerable difficulty has been encountered in manual operations.
10. In open air welding operations, means must be provided for shielding the root or back of the welds. Backing fixtures are often used for this purpose. In one type, an auxiliary supply of inert gas is provided to shield the back of the weld. In the other, a solid or grooved backing bar fits tightly against the back of the weld and provides the required shielding. Fixtures which provide an inert gas shield are preferred, especially in manual welding operations with low welding speeds. Figure 7-18 shows backing fixtures used in butt welding heavy plate and thin sheet, respectively. Similar types of fixtures are used for other joint designs. However, the design of the fixtures varies with the design of the joints. For fillet welds on tee joints, shielding should be supplied for two sides of the weld in addition to shielding the face of the weld.
11. For some applications, it may be easier to enclose the back of the weld, as in a tank, and supply inert gas for shielding purposes. This method is necessary in welding tanks, tubes, or other enclosed structures where access to the back of the weld is not possible. In some weldments, it may be necessary to machine holes or grooves in the structures in order to provide shielding gas for the back or root of the welds.
WARNING
When using weld backup tape, the weld must be allowed to cool for several minutes before attempting to remove the tape from the workpiece.
12. Use of backing fixtures such as shown in figure 7-18 can be eliminated in many cases by the use of weld backup tape. This tape consists of a center strip of heat resistant fiberglass adhered to a wider strip of aluminum foil, along with a strip of adhesive on each side of the center strip that is used to hold tape to the underside of the tack welded joint. During the welding, the fiberglass portion of the tape is in direct contact with the molten metal, preventing excessive penetration. Contamination or oxidation of the underside of the weld is prevented by the airtight seal created by the aluminum foil strip. The tape can be used on butt or corner joints (fig. 7-19) or, because of its flexibility, on curved or irregularly shaped surfaces. The surface to which the tape is applied must be clean and dry. Best results are obtained by using a root gap wide enough to allow full penetration.
13. Bend or notch toughness tests are the best methods for evaluating shielding conditions, but visual inspection of the weld surface, which is not an infallible method, is the only nondestructive means for evaluating weld quality at the present time. With this method, the presence of a heavy gray scale with a nonmetallic luster on the weld bead indicates that the weld has been contaminate badly and has low ductility. Also, the weld surface may be shiny but have different colors, ranging from grayish blue to violet to brown. This type of discoloration may be found on severely contaminated welds or may be due only to surface contamination, while the weld itself may be satisfactory. However, the quality of the weld cannot be determined without a destructive test. With good shielding procedures, weld surfaces are shiny and show no discoloration.
(3) Welding chambers.
(a) For some applications, inert gas filled welding chambers are used. The advantage of using such chambers is that good shielding may be obtained for the root and face of the weld without the use of special fixtures. Also, the surface appearance of such welds is a fairly reliable measure of shielding conditions. The use of chambers is especially advantageous when complex joints are being welded. However, chambers are not required for many applications, and their use may be limited.
(b) Welding chambers vary in size and shape, depending on their use and the size of assemblies to be welded. The inert atmospheres maybe obtained by evacuating the chamber and filling it with helium or argon, purging the chamber with inert gas, or collapsing the chamber to expel air and refilling it with an inert gas. Plastic bags have been used in this latter manner. When the atmospheres are obtained by purging or collapsing the chambers, inert gas usually is supplied through the welding torch to insure complete protection of the welds.
(4) Joint designs. Joint designs for titanium are similar to those used for other metals. For welding a thin sheet, the tungsten-arc process generally is used. With this process, butt welds may be made with or without filler rod, depending on the thickness of the joint and fitup. Special shearing procedures sometimes are used so that the root opening does not exceed 8 percent of the sheet thickness. If fitup is this good, filler rod is not required. If fitup is not this good, filler metal is added to obtain full thickness joints. In welding thicker sheets (greater than 0.09 in. (2.3 mm)), both the tungsten-arc and consumable electrode processes are used with a root opening. For welding titanium plates, bars, or forgings, both the tungsten-arc and consumable electrode processes also are used with single and double V joints. In all cases, good weld penetration may be obtained with excessive drop through. However, penetration and dropthrough are controlled more easily by the use of proper backing fixtures.
NOTE
Because of the low thermal conductivity of titanium, weld beads tend to be wider than normal. However, the width of the beads is generally controlled by using short arc lengths, or by placing chill bars or the clamping toes of the jig close to the sides of the joints.
(5) Welding variables.
(a) Welding speed and current for titanium alloys depend on the process used, shielding gas, thickness of the material being welded, design of the backing fixtures, along with the spacing of chill bars or clamping bars in the welding jig. Welding speeds vary from about 3.0 to 40.0 in. (76.2 to 1016.0 mm) per minute. The highest welding speeds are obtained with the consumable electrode process. In most cases, direct current is used with straight polarity for the tungsten-arc process. Reverse polarity is used for the consumable electrode process.
(b) Arc wander has proven troublesome in some automatic welding operations. With arc wander, the arc from the tungsten or consumable electrode moves from one side of the weld joint to the other side. A straight, uniform weld bead will not be produced. Arc wander is believed to be caused by magnetic disturbances, bends in the filler wire, coatings on the filler wire, or a combination of these. Special metal shields and wire straighteners have been used to overcome arc wander, but have not been completely satisfactory. Also, constant voltage welding machines have been used in an attempt to overcome this problem. These machines also have not been completely satisfactory.
(c) In setting up arc welding operations for titanium, the welding conditions should be evaluated on the basis of weld joint properties and appearance. Radiographs will show if porosity or cracking is present in the weld joint. A simple bend test or notch toughness test will show whether or not the shielding conditions are adequate. A visual examination of the weld will show if the weld penetration and contour are satisfactory. After adequate procedures are established, careful controls are desirable to ensure that the shielding conditions are not changed.
(6) Weld defects.
(a) General. Defects in arc welded joints in titanium alloys consist mainly of porosity (see (b)) and cold cracks (see (c)). Weld penetration can be controlled by adjusting welding conditions.
(b) Porosity. Weld porosity is a major problem in arc welding titanium alloys. Although acceptable limits for porosity in arc welded joints have not been establish, porosity has been observed in tungsten-arc welds in practically all of the alloys which appear suitable for welding operations. It does not extend to the surface of the weld, but has been detected in radiographs. It usually occurs close to the fusion line of the welds. Weld porosity may be reduced by agitating the molten weld puddle and adjusting welding speeds. Also, remelting the weld will eliminate some of the porosity present after the first pass. However, the latter method of reducing weld porosity tends to increase weld contamination.
(c) Cracks.
1. With adequate shielding procedures and suitable alloys, cracks should not be a problem. However, cracks have been troublesome in welding some alloys. Weld cracks are attributed to a number of causes. In commercially pure titanium, weld metal cracks are believed to be caused by excessive oxygen or nitrogen contamination. These cracks are usually observed in weld craters. In some of the alpha-beta alloys, transverse cracks in the weld metal and heat affected zones are believed to be due to the low ductility of the weld zones. However, cracks in these alloys also may be due to contamination. Cracks also have been observed in alpha-beta welds made under restraint and with high external stresses. These cracks are sometimes attributed to the hydrogen content of the alloys.
NOTE
If weld cracking is due to contamination, it may be controlled by improving shielding conditions. However, repair welding on excessively contaminated welds is not practical in many cases.
2. Cracks which are caused by the low ductility of welds in alpha-beta alloys can be prevented by heat treating or stress relieving the weldment in a furnance immediately after welding. Oxyacetylene torches also have been used for this purpose. However, care must be taken so that the weldment is not overheated or excessively contaminated by the torch heating operation.
3. Cracks due to hydrogen may be prevented by vacuum annealing treatments prior to welding.
(7) Availability of welding filler wire. Most of the titanium alloys which are being used in arc welding applications are available as wire for use as welding filler metal. These alloys are listed below:
(a) Commercially pure titanium --commercially available as wire.
(b) Ti-5A1-2-1/2Sn alloy --available as wire in experimental quantities.
(c) Ti-1-1/2A1-3Mn alloy --available as wire in experimental quantities.
(d) Ti-6A1-4V alloy --available as wire in experimental quantities.
(e) There has not been a great deal of need for the other alloys as welding filler wires. However, if such a need occurs, most of these alloys also could be reduced to wire. In fact, the Ti-8Mn alloy has been furnished as welding wire to meet some requests.
d. Pressure Welding. Solid phase or pressure welding has been used to join titanium and titanium alloys. In these processes, the surfaces to be jointed are not melted. They are held together under pressure and heated to elevated temperatures (900 to 2000°F (482 to 1093°C)). One method of heating used in pressure welding is the oxyacetylene flame. With suitable pressure and upset, good welds are obtainable in the high strength alpha-beta titanium alloys. The contaminated area on the surface of the weld is displaced from the joint area by the upset, which occurs during welding. This contaminated surface is machined off after welding. Another method of heating is by heated dies. Strong lap joints are obtained with this method in commercially pure titanium and a high strength alpha-beta alloy. By heating in this manner, welds may be made in very short periods of time, and inert gas shielding may be supplied to the joint. With all of the heating methods, less than 2 minutes is required to complete the welding operation. With solid phase or pressure welding processes, it is possible to produce ductile welds in the high strength alpha-beta alloys by using temperatures which do not cause embrittlement in these alloys.
7-22. NICKEL AND MONTEL WELDING
a. General. Nickel is a hard, malleable, ductile metal. Nickel and its alloys are commonly used when corrosion resistance is required. Nickel and nickel alloys such as Monel can, in general, be welded by metal-arc and gas welding methods. Some nickel alloys are more difficult to weld due to different compositions. The operator should make trial welds with reverse polarity at several current values and select the one best suited for the work. Generally, the oxyacetylene welding methods are preferred for smaller plates. However, small plates can be welded by the metal-arc and carbon-arc processes, and large plates are most satisfactorily joined, especially if the plate is nickel clad steel.
When welding, the nickel alloys can be treated much in the same manner as austenitic stainless steels with a few exceptions. These exceptions are:
(1) The nickel alloys will acquire a surface or coating which melts at a temperature approximately 1000°F (538°C) above the melting point of the base metal.
(2) The nickel alloys are susceptible to embrittlement at welding temperatures by lead, sulfur, phosphorus, and some low-temperature metals and alloys.
(3) Weld penetration is less than expected with other metals.
When compensation is made for these three factors, the welding procedures used for the nickel alloys can he the same as those used for stainless steel. This is because the melting point, the coefficient of thermal expansion, and the thermal conductivity are similar to austenitic stainless steel.
It is necessary that each of these precautions be considered. The surface oxide should be completely removed from the joint area by grinding, abrasive blasting, machining, or by chemical means. When chemical etches are used, they must be completely removed by rinsing prior to welding. The oxide which melts at temperatures above the melting point of the base metal may enter the weld as a foreign material, or impurity, and will greatly reduce the strength and ductility of the weld. The problem of embrittlement at welding temperatures also means that the weld surface must be absolutely clean. Paints, crayon markings, grease, oil, machining lubricants, and cutting oils may all contain the ingredients which will cause embrittlement. They must be completely removed for the weld area to avoid embrittlement. It is necessary to increase the opening of groove angles and to provide adequate root openings when full-penetration welds are used. The bevel or groove angles should be increased to approximately 40 percent over those used for carbon steel.
b. Joint Design. Butt joints are preferred but corner and lap joints can be effectively welded. Beveling is not required on plates 1/16 to 1/8 in. (1.6 to 3.2 mm) thick. With thicker materials, a bevel angle of 35 to 37-1/2 degrees should be made. When welding lap joints, the weld should be made entirely with nickel electrodes if water or air tightness is required.
c. Welding Techniques.
(1) Clean all surfaces to be welded either mechanically by machine, sand-blasting, grinding, or with abrasive cloth; or chemically by pickling.
(2) Plates having U or V joints should be assembled, and if nickel clad steel, should be tacked on the steel side to prevent warping and distortion. After it is determined that the joint is even and flat, complete the weld on the steel side. Chip out and clean the nickel side and weld. If the base metal on both sides is nickel, clean out the groove on the unwelded side prior to beginning the weld on that side.
(3) If desired, the nickel side may be completed first. However, the steel side must be tacked and thoroughly cleaned and beveled (or gouged) down to the root of the nickel weld prior to welding.
(4) Lap and corner joints are successfully welded by depositing a bead of nickel metal into the root and then weaving successive beads over the root weld.
(5) The arc drawn for nickel or nickel alloy welding should be slightly shorter than that used in normal metal-arc welding. A 1/16 to 1/8 in. (1.6 to 3.2 mm) arc is a necessity.
(6) Any position weld can be accomplished that can be satisfactorily welded by normal metal-arc welding of steel.
d. Welding Methods.
(1) Almost all the welding processes can be used for welding the nickel alloys. In addition, they can be joined by brazing and soldering.
(2) Welding nickel alloys. The most popular processes for welding nickel alloys are the shielded metal arc welding process, the gas tungsten arc welding process, and the gas metal arc welding process. Process selection depends on the normal factors. When shielded metal arc welding is used the procedures are essentially the same as those used for stainless steel welding.
The welding procedure schedule for using gas tungsten arc welding (TIG) is shown by table 7-29. The welding procedure schedule for gas metal arc welding (MIG) is shown by table 7-30. The procedure information set forth on these tables will provide starting points for developing the welding procedures.
(3) No postweld heat treatment is required to maintain or restore corrosion resistance of the nickel alloys. Heat treatment is required for precipitating hardening alloys. Stress relief may be required to meet certain specifications to avoid stress corrosion cracking in applications involving hydrofluoric acid vapors or caustic solutions.